N-Acetylcysteine for Pediatric Obsessive-Compulsive Disorder: A Small Pilot Study
- PMID: 31800306
- PMCID: PMC7133418
- DOI: 10.1089/cap.2019.0041
N-Acetylcysteine for Pediatric Obsessive-Compulsive Disorder: A Small Pilot Study
Abstract
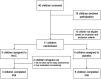
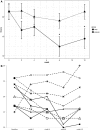
Background: Many children and adults with Obsessive-Compulsive Disorder (OCD) fail to respond to first-line pharmacological and behavioral treatments. Glutamate dysfunction may contribute to the development of OCD. N-acetylcysteine (NAC), a glutamate modulating drug, has shown to be a promising agent in adults with OCD. Methods: We conducted a double-blind, placebo-controlled clinical trial from July 2012 to January 2017. Children ages 8 to 17 years with OCD were assigned to receive NAC (up to 2700 mg/day) or the matching placebo for a period of 12 weeks. Children were required to be on stable psychiatric treatment (both medication and therapy) but were not required to be treatment-refractory. The primary outcome was OCD symptom severity as measured by the Children's Yale-Brown Obsessive-Compulsive Scale (CY-BOCS). We used linear mixed models to analyze the effect of NAC compared to placebo. Results: Due to poor recruitment and eventual expiration of the study medication, enrollment was stopped at 11 children out of a planned sample size of 40. Nonetheless, NAC was associated with significant reduction in CY-BOCS total score compared to placebo (Satterthwaite's test: t (37) = 2.36, p = 0.024) with effects separating from placebo beginning at week 8. Mean CY-BOCS total score decreased in the NAC group from 21.4 ± 4.65 at baseline to 14.4 ± 5.55 at week 12. In the placebo group, mean CY-BOCS total score remained unchanged (21.3 ± 4.65). In the NAC group, 1 out of 5 participants achieved >35% improvement in CY-BOCS total score, while none of the six patients in placebo group reached this improvement level. NAC and placebo were well tolerated. One mild adverse event was reported in each group. Conclusions: Our trial suggests that there may be some initial improvement in OCD symptom severity with NAC treatment. NAC was well tolerated in the study population. Future trials should employ multiple sites and have a larger study population to further confirm any benefits of NAC.
Keywords: N-acetylcysteine; adolescence; child; obsessive-compulsive disorder; randomized controlled trial.
Conflict of interest statement
No competing financial interests exist.
Figures
Similar articles
-
Randomized, Double-Blind, Placebo-Controlled Trial of N-Acetylcysteine Augmentation for Treatment-Resistant Obsessive-Compulsive Disorder.J Clin Psychiatry. 2017 Jul;78(7):e766-e773. doi: 10.4088/JCP.16m11101. J Clin Psychiatry. 2017. PMID: 28617566 Clinical Trial.
-
N-Acetyl Cysteine (NAC) in the Treatment of Obsessive-Compulsive Disorder: A 16-Week, Double-Blind, Randomised, Placebo-Controlled Study.CNS Drugs. 2015 Sep;29(9):801-9. doi: 10.1007/s40263-015-0272-9. CNS Drugs. 2015. PMID: 26374743 Clinical Trial.
-
N-acetylcysteine augmentation therapy for moderate-to-severe obsessive-compulsive disorder: randomized, double-blind, placebo-controlled trial.J Clin Pharm Ther. 2016 Apr;41(2):214-9. doi: 10.1111/jcpt.12370. Epub 2016 Mar 2. J Clin Pharm Ther. 2016. PMID: 26931055 Clinical Trial.
-
"N-Acetylcysteine for Obsessive-Compulsive and Related Disorders in Children and Adolescents: A Review".Ann Pharmacother. 2023 Jul;57(7):847-854. doi: 10.1177/10600280221138092. Epub 2022 Nov 16. Ann Pharmacother. 2023. PMID: 36384314 Review.
-
A systematic review and meta-analysis: Memantine augmentation in moderate to severe obsessive-compulsive disorder.Psychiatry Res. 2019 Dec;282:112602. doi: 10.1016/j.psychres.2019.112602. Epub 2019 Oct 4. Psychiatry Res. 2019. PMID: 31630042
Cited by
-
Efficacy of N-acetylcysteine plus pirfenidone in the treatment of idiopathic pulmonary fibrosis: a systematic review and meta-analysis.BMC Pulm Med. 2023 Nov 29;23(1):479. doi: 10.1186/s12890-023-02778-w. BMC Pulm Med. 2023. PMID: 38031002 Free PMC article.
-
Early Identification and Intervention in Pediatric Obsessive-Compulsive Disorder.Brain Sci. 2023 Feb 25;13(3):399. doi: 10.3390/brainsci13030399. Brain Sci. 2023. PMID: 36979207 Free PMC article. Review.
-
Clinical efficacy and quality of life effect of acetylcysteine plus pirfenidone in patients with pulmonary fibrosis.Am J Transl Res. 2022 Aug 15;14(8):5660-5668. eCollection 2022. Am J Transl Res. 2022. PMID: 36105040 Free PMC article.
-
Glutamatergic Medications for Obsessive-Compulsive and Related Disorders: A Systematic Review and Meta-Analysis.JAMA Netw Open. 2025 Jan 2;8(1):e2452963. doi: 10.1001/jamanetworkopen.2024.52963. JAMA Netw Open. 2025. PMID: 39745698 Free PMC article.
-
N-Acetylcysteine Mitigates Social Dysfunction in a Rat Model of Autism Normalizing Glutathione Imbalance and the Altered Expression of Genes Related to Synaptic Function in Specific Brain Areas.Front Psychiatry. 2022 Feb 25;13:851679. doi: 10.3389/fpsyt.2022.851679. eCollection 2022. Front Psychiatry. 2022. PMID: 35280167 Free PMC article.
References
-
- Afshar H, Roohafza H, Mohammad-Beigi H, Haghighi M, Jahangard L, Shokouh P, Sadeghi M, Hafezian H: N-acetylcysteine add-on treatment in refractory obsessive-compulsive disorder: A randomized, double-blind, placebo-controlled trial. J Clin Psychopharmacol 32:797–803, 2012 - PubMed
-
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed Washington, DC: American Psychiatric Association; 2013
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

