Immune Checkpoint Inhibitor Therapy in Patients With Preexisting Inflammatory Bowel Disease
- PMID: 31800340
- PMCID: PMC7030892
- DOI: 10.1200/JCO.19.01674
Immune Checkpoint Inhibitor Therapy in Patients With Preexisting Inflammatory Bowel Disease
Abstract
Purpose: The risk of immune checkpoint inhibitor therapy-related GI adverse events in patients with cancer and inflammatory bowel disease (IBD) has not been well described. We characterized GI adverse events in patients with underlying IBD who received immune checkpoint inhibitors.
Patients and methods: We performed a multicenter, retrospective study of patients with documented IBD who received immune checkpoint inhibitor therapy between January 2010 and February 2019. Backward selection and multivariate logistic regression were conducted to assess risk of GI adverse events.
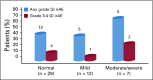
Results: Of the 102 included patients, 17 received therapy targeting cytotoxic T-lymphocyte antigen-4, and 85 received monotherapy targeting programmed cell death 1 or its ligand. Half of the patients had Crohn's disease, and half had ulcerative colitis. The median time from last active IBD episode to immunotherapy initiation was 5 years (interquartile range, 3-12 years). Forty-three patients were not receiving treatment of IBD. GI adverse events occurred in 42 patients (41%) after a median of 62 days (interquartile range, 33-123 days), a rate higher than that among similar patients without underlying IBD who were treated at centers participating in the study (11%; P < .001). GI events among patients with IBD included grade 3 or 4 diarrhea in 21 patients (21%). Four patients experienced colonic perforation, 2 of whom required surgery. No GI adverse event-related deaths were recorded. Anti-cytotoxic T-lymphocyte antigen-4 therapy was associated with increased risk of GI adverse events on univariable but not multivariable analysis (odds ratio, 3.19; 95% CI, 1.8 to 9.48; P = .037; and odds ratio, 4.72; 95% CI, 0.95 to 23.53; P = .058, respectively).
Conclusion: Preexisting IBD increases the risk of severe GI adverse events in patients treated with immune checkpoint inhibitors.
Figures

Comment in
-
Meaningful Symptoms of Immune Checkpoint Inhibitor Therapy-Related Gastrointestinal Adverse Events in Patients With Inflammatory Bowel Disease.J Clin Oncol. 2020 May 20;38(15):1748-1749. doi: 10.1200/JCO.20.00033. Epub 2020 Apr 6. J Clin Oncol. 2020. PMID: 32250716 No abstract available.
-
Reply to Y. Inagaki et al.J Clin Oncol. 2020 May 20;38(15):1749-1750. doi: 10.1200/JCO.20.00295. Epub 2020 Apr 6. J Clin Oncol. 2020. PMID: 32250720 No abstract available.
References
-
- Baumeister SH, Freeman GJ, Dranoff G, et al. Coinhibitory pathways in immunotherapy for cancer. Annu Rev Immunol. 2016;34:539–573. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

