Dead Cas Systems: Types, Principles, and Applications
- PMID: 31801211
- PMCID: PMC6929090
- DOI: 10.3390/ijms20236041
Dead Cas Systems: Types, Principles, and Applications
Abstract
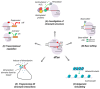
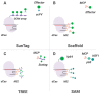
The gene editing tool CRISPR-Cas has become the foundation for developing numerous molecular systems used in research and, increasingly, in medical practice. In particular, Cas proteins devoid of nucleolytic activity (dead Cas proteins; dCas) can be used to deliver functional cargo to programmed sites in the genome. In this review, we describe current CRISPR systems used for developing different dCas-based molecular approaches and summarize their most significant applications. We conclude with comments on the state-of-art in the CRISPR field and future directions.
Keywords: Cas9; cancer; chromatin; dCas; editing; epigenetics; hereditary diseases; infectious diseases; inflammatory diseases; transcription.
Conflict of interest statement
The authors declare that they have no potential conflicts of interests.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

