Bone Pain in Cancer Patients: Mechanisms and Current Treatment
- PMID: 31801267
- PMCID: PMC6928918
- DOI: 10.3390/ijms20236047
Bone Pain in Cancer Patients: Mechanisms and Current Treatment
Abstract
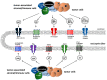
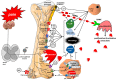
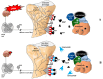
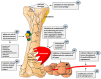
The skeletal system is the third most common site for cancer metastases, surpassed only by the lungs and liver. Many tumors, especially those of the breast, prostate, lungs, and kidneys, have a strong predilection to metastasize to bone, which causes pain, hypercalcemia, pathological skeletal fractures, compression of the spinal cord or other nervous structures, decreased mobility, and increased mortality. Metastatic cancer-induced bone pain (CIBP) is a type of chronic pain with unique and complex pathophysiology characterized by nociceptive and neuropathic components. Its treatment should be multimodal (pharmacological and non-pharmacological), including causal anticancer and symptomatic analgesic treatment to improve quality of life (QoL). The aim of this paper is to discuss the mechanisms involved in the occurrence and persistence of cancer-associated bone pain and to review the treatment methods recommended by experts in clinical practice. The final part of the paper reviews experimental therapeutic methods that are currently being studied and that may improve the efficacy of bone pain treatment in cancer patients in the future.
Keywords: cancer-induced bone pain; multimodal pain treatment; neuropathic pain; nociceptive pain.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

