Endogenous Retroviruses Activity as a Molecular Signature of Neurodevelopmental Disorders
- PMID: 31801288
- PMCID: PMC6928979
- DOI: 10.3390/ijms20236050
Endogenous Retroviruses Activity as a Molecular Signature of Neurodevelopmental Disorders
Abstract
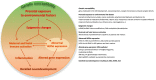
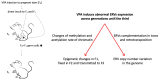
Human endogenous retroviruses (HERVs) are genetic elements resulting from relics of ancestral infection of germline cells, now recognized as cofactors in the etiology of several complex diseases. Here we present a review of findings supporting the role of the abnormal HERVs activity in neurodevelopmental disorders. The derailment of brain development underlies numerous neuropsychiatric conditions, likely starting during prenatal life and carrying on during subsequent maturation of the brain. Autism spectrum disorders, attention deficit hyperactivity disorders, and schizophrenia are neurodevelopmental disorders that arise clinically during early childhood or adolescence, currently attributed to the interplay among genetic vulnerability, environmental risk factors, and maternal immune activation. The role of HERVs in human embryogenesis, their intrinsic responsiveness to external stimuli, and the interaction with the immune system support the involvement of HERVs in the derailed neurodevelopmental process. Although definitive proofs that HERVs are involved in neurobehavioral alterations are still lacking, both preclinical models and human studies indicate that the abnormal expression of ERVs could represent a neurodevelopmental disorders-associated biological trait in affected individuals and their parents.
Keywords: ASD animal model.; autism; endogenous retroviruses (ERVs); maternal immune activation; neurodevelopmental disorders.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

