Does the use of a closed-suction drain reduce the effectiveness of an antibiotic-loaded spacer in two-stage exchange Arthroplasty for Periprosthetic hip infection? A prospective, randomized, controlled study
- PMID: 31801510
- PMCID: PMC6894212
- DOI: 10.1186/s12891-019-2974-5
Does the use of a closed-suction drain reduce the effectiveness of an antibiotic-loaded spacer in two-stage exchange Arthroplasty for Periprosthetic hip infection? A prospective, randomized, controlled study
Abstract
Background: There is a concern regarding the use of a closed-suction drain (CSD) in two-stage exchange arthroplasty for periprosthetic joint infection as it may decrease the antibiotic concentrations in the joint fluids. The purpose of this study was to identify whether the use of a CSD could reduce local antibiotic concentrations following spacer implantation.
Methods: A prospective, randomized, controlled trial was conducted at our institution between January 2018 and November 2018. We enrolled 32 patients undergoing two-stage exchange arthroplasty for periprosthetic hip infection with an interim cement spacer containing 4-g vancomycin and 2-g meropenem per 40-g methyl-methacrylate cement polymer. Patients were randomized and evenly divided into the study group (non-CSD) and control group (CSD group) by sealed envelopes. Drainage samples of joint fluids (n = 160) were collected every 24 h for the first five days following spacer implantation. The antibiotic concentrations of drainage samples were measured by high-performance liquid chromatography, and the bioactivities of the drainage samples against methicillin-sensitive and methicillin-resistant Staphylococcus aureus (MSSA and MRSA) and E. coli were assessed.
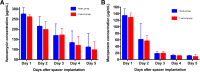
Results: There was no significant difference in the decrease of vancomycin (study group vs. control group: 163.20 ± 77.05 vs. 162.39 ± 36.31; p = 0.917) and meropenem concentration (123.78 ± 21.04 vs. 117.27 ± 19.38; P = 0.548) between the two groups during the first five days following spacer implantation. All joint drainage samples in each group exhibited antibacterial activity against MSSA, MRSA and E. coli.
Conclusions: The use of CSD following the implantation of an antibiotic-loaded cement spacer does not reduce the effectiveness of such a spacer in two-stage exchange arthroplasty. (Chinese Clinical Trial Registry, ChiCTR-INR-17014162. Registered 26 December 2017.).
Keywords: Antibiotic concentration; Antibiotic-loaded cement spacer; Closed-suction drain; Periprosthetic joint infection; Two-staged exchange arthroplasty.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Antibiotics release from cement spacers used for two-stage treatment of implant-associated infections after total joint arthroplasty.J Biomed Mater Res B Appl Biomater. 2019 Jul;107(5):1587-1597. doi: 10.1002/jbm.b.34251. Epub 2018 Oct 12. J Biomed Mater Res B Appl Biomater. 2019. PMID: 30312529 Free PMC article.
-
Two stage revision hip arthroplasty in periprosthetic joint infection. Comparison study: with or without the use of a spacer.Int Orthop. 2017 Nov;41(11):2253-2258. doi: 10.1007/s00264-017-3500-8. Epub 2017 May 18. Int Orthop. 2017. PMID: 28516223
-
The Influence of Spacer Design on the Rate of Complications in Two-Stage Revision Hip Arthroplasty.J Arthroplasty. 2019 Jun;34(6):1201-1206. doi: 10.1016/j.arth.2019.02.012. Epub 2019 Feb 15. J Arthroplasty. 2019. PMID: 30879874
-
Two-stage approach to total knee arthroplasty using colistin-loaded articulating cement spacer for vancomycin-resistant Pseudomonas aeruginosa infection in an arthritic knee.Eur J Orthop Surg Traumatol. 2019 Jan;29(1):227-230. doi: 10.1007/s00590-018-2268-x. Epub 2018 Jun 18. Eur J Orthop Surg Traumatol. 2019. PMID: 29915953 Review.
-
Antibiotic-impregnated PMMA hip spacers: Current status.Acta Orthop. 2006 Aug;77(4):628-37. doi: 10.1080/17453670610012719. Acta Orthop. 2006. PMID: 16929441 Review.
Cited by
-
Design Characteristics and Recruitment Rates for Randomized Trials of Peri-Prosthetic Joint Infection Management: A Systematic Review.Antibiotics (Basel). 2023 Sep 27;12(10):1486. doi: 10.3390/antibiotics12101486. Antibiotics (Basel). 2023. PMID: 37887189 Free PMC article. Review.
-
Value of closed suction drainage in arthroscopic and minimally invasive surgery of the ankle joint: a prospective randomised study.Arch Orthop Trauma Surg. 2023 Feb;143(2):657-663. doi: 10.1007/s00402-021-04107-4. Epub 2021 Aug 16. Arch Orthop Trauma Surg. 2023. PMID: 34401935 Clinical Trial.
-
Negative pressure wound therapy for surgical wounds healing by primary closure.Cochrane Database Syst Rev. 2022 Apr 26;4(4):CD009261. doi: 10.1002/14651858.CD009261.pub7. Cochrane Database Syst Rev. 2022. PMID: 35471497 Free PMC article.
-
Development of Training Videos Based on Virtual Reality Side-Cut High-Simulation Simulator of Human Wound Technology and Sealed Sputum Suction.J Healthc Eng. 2021 Sep 8;2021:9985041. doi: 10.1155/2021/9985041. eCollection 2021. J Healthc Eng. 2021. Retraction in: J Healthc Eng. 2023 May 24;2023:9754127. doi: 10.1155/2023/9754127. PMID: 34540192 Free PMC article. Retracted.
References
-
- Hsu Yung-Heng, Hu Chih-chien, Hsieh Pang-Hsin, Shih Hsin-Nung, Ueng Steve W.N., Chang Yuhan. Vancomycin and Ceftazidime in Bone Cement as a Potentially Effective Treatment for Knee Periprosthetic Joint Infection. The Journal of Bone and Joint Surgery. 2017;99(3):223–231. doi: 10.2106/JBJS.16.00290. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical