Determinants of high residual post-PCV13 pneumococcal vaccine-type carriage in Blantyre, Malawi: a modelling study
- PMID: 31801542
- PMCID: PMC6894346
- DOI: 10.1186/s12916-019-1450-2
Determinants of high residual post-PCV13 pneumococcal vaccine-type carriage in Blantyre, Malawi: a modelling study
Abstract
Background: In November 2011, Malawi introduced the 13-valent pneumococcal conjugate vaccine (PCV13) into the routine infant schedule. Four to 7 years after introduction (2015-2018), rolling prospective nasopharyngeal carriage surveys were performed in the city of Blantyre. Carriage of Streptococcus pneumoniae vaccine serotypes (VT) remained higher than reported in high-income countries, and impact was asymmetric across age groups.
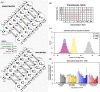
Methods: A dynamic transmission model was fit to survey data using a Bayesian Markov-chain Monte Carlo approach, to obtain insights into the determinants of post-PCV13 age-specific VT carriage.
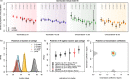
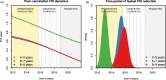
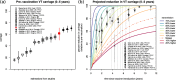
Results: Accumulation of naturally acquired immunity with age and age-specific transmission potential were both key to reproducing the observed data. VT carriage reduction peaked sequentially over time, earlier in younger and later in older age groups. Estimated vaccine efficacy (protection against carriage) was 66.87% (95% CI 50.49-82.26%), similar to previous estimates. Ten-year projected vaccine impact (VT carriage reduction) among 0-9 years old was lower than observed in other settings, at 76.23% (CI 95% 68.02-81.96%), with sensitivity analyses demonstrating this to be mainly driven by a high local force of infection.
Conclusions: There are both vaccine-related and host-related determinants of post-PCV13 pneumococcal VT transmission in Blantyre with vaccine impact determined by an age-specific, local force of infection. These findings are likely to be generalisable to other Sub-Saharan African countries in which PCV impact on carriage (and therefore herd protection) has been lower than desired, and have implications for the interpretation of post-PCV carriage studies and future vaccination programs.
Keywords: Intervention; Malawi; Modelling; Pneumococcus; pcv13.
Conflict of interest statement
Dr. Bar-Zeev reports investigator-initiated research grants from GlaxoSmithKline Biologicals and from Takeda Pharmaceuticals outside the submitted work. The authors declare that they have no competing interests.
Figures

References
-
- Brown J, Hammerschmidt S, Orihuela C, editors. Streptococcus Pneumoniae: molecular mechanisms of host-pathogen interactions. 1st ed: Elsevier; 2015. 10.1016/C2012-0-00722-3.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

