Molecular Mechanisms of Conidial Germination in Aspergillus spp
- PMID: 31801804
- PMCID: PMC6903801
- DOI: 10.1128/MMBR.00049-19
Molecular Mechanisms of Conidial Germination in Aspergillus spp
Abstract
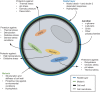
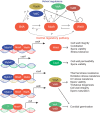
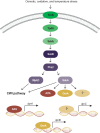
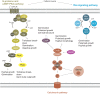
Aspergilli produce conidia for reproduction or to survive hostile conditions, and they are highly effective in the distribution of conidia through the environment. In immunocompromised individuals, inhaled conidia can germinate inside the respiratory tract, which may result in invasive pulmonary aspergillosis. The management of invasive aspergillosis has become more complex, with new risk groups being identified and the emergence of antifungal resistance. Patient survival is threatened by these developments, stressing the need for alternative therapeutic strategies. As germination is crucial for infection, prevention of this process might be a feasible approach. A broader understanding of conidial germination is important to identify novel antigermination targets. In this review, we describe conidial resistance against various stresses, transition from dormant conidia to hyphal growth, the underlying molecular mechanisms involved in germination of the most common Aspergillus species, and promising antigermination targets. Germination of Aspergillus is characterized by three morphotypes: dormancy, isotropic growth, and polarized growth. Intra- and extracellular proteins play an important role in the protection against unfavorable environmental conditions. Isotropically expanding conidia remodel the cell wall, and biosynthetic machineries are needed for cellular growth. These biosynthetic machineries are also important during polarized growth, together with tip formation and the cell cycle machinery. Genes involved in isotropic and polarized growth could be effective antigermination targets. Transcriptomic and proteomic studies on specific Aspergillus morphotypes will improve our understanding of the germination process and allow discovery of novel antigermination targets and biomarkers for early diagnosis and therapy.
Keywords: Aspergillus; conidia; dormant; germination; isotropic growth; polarized growth.
Copyright © 2019 American Society for Microbiology.
Figures


References
-
- McCartney A, West J. 2007. Dispersal of fungal spores through the air, p 65–81 In Samson RA, Dijksterhuis J (ed), Food mycology: a multifaceted approach to fungi and food. CRC Press, Boca Raton, FL.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

