DNA polymerase β nucleotide-stabilized template misalignment fidelity depends on local sequence context
- PMID: 31801827
- PMCID: PMC6956524
- DOI: 10.1074/jbc.RA119.010594
DNA polymerase β nucleotide-stabilized template misalignment fidelity depends on local sequence context
Abstract
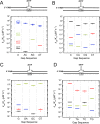
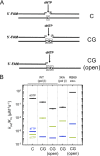
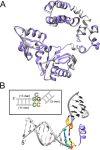
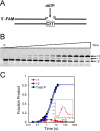
DNA polymerase β has two DNA-binding domains that interact with the opposite sides of short DNA gaps. These domains contribute two activities that modify the 5' and 3' margins of gapped DNA during base excision repair. DNA gaps greater than 1 nucleotide (nt) pose an architectural and logistical problem for the two domains to interact with their respective DNA termini. Here, crystallographic and kinetic analyses of 2-nt gap-filling DNA synthesis revealed that the fidelity of DNA synthesis depends on local sequence context. This was due to template dynamics that altered which of the two template nucleotides in the gap served as the coding nucleotide. We observed that, when a purine nucleotide was in the first coding position, DNA synthesis fidelity was similar to that observed with a 1-nt gap. However, when the initial templating nucleotide was a pyrimidine, fidelity was decreased. If the first templating nucleotide was a cytidine, there was a significantly higher probability that the downstream template nucleotide coded for the incoming nucleotide. This dNTP-stabilized misalignment reduced base substitution and frameshift deletion fidelities. A crystal structure of a binary DNA product complex revealed that the cytidine in the first templating site was in an extrahelical position, permitting the downstream template nucleotide to occupy the coding position. These results indicate that DNA polymerase β can induce a strain in the DNA that modulates the position of the coding nucleotide and thereby impacts the identity of the incoming nucleotide. Our findings demonstrate that "correct" DNA synthesis can result in errors when template dynamics induce coding ambiguity.
Keywords: DNA polymerase; DNA repair; DNA structure; DNA synthesis; X-ray crystallography; base excision repair; kinetics; mutagenesis; mutagenesis mechanism; polymerase fidelity; structure–function.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health
Figures


References
-
- Prasad R., Beard W. A., and Wilson S. H. (1994) Studies of gapped DNA substrate binding by mammalian DNA polymerase β: dependence on 5′-phosphate group. J. Biol. Chem. 269, 18096–18101 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

