Monoclonal Antibodies with Neutralizing Activity and Fc-Effector Functions against the Machupo Virus Glycoprotein
- PMID: 31801871
- PMCID: PMC7022345
- DOI: 10.1128/JVI.01741-19
Monoclonal Antibodies with Neutralizing Activity and Fc-Effector Functions against the Machupo Virus Glycoprotein
Abstract
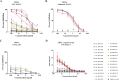
Machupo virus (MACV), the causative agent of Bolivian hemorrhagic fever (BHF), is a New World arenavirus that was first isolated in Bolivia from a human spleen in 1963. Due to the lack of a specific vaccine or therapy, this virus is considered a major risk to public health and is classified as a category A priority pathogen by the U.S. National Institutes of Health. In this study, we used DNA vaccination against the MACV glycoprotein precursor complex (GPC) and murine hybridoma technology to generate 25 mouse monoclonal antibodies (MAbs) against the GPC of MACV. Out of 25 MAbs, five were found to have potent neutralization activity in vitro against a recombinant vesicular stomatitis virus expressing MACV GPC (VSV-MACV) as well as against authentic MACV. Furthermore, the five neutralizing MAbs exhibited strong antibody-dependent cellular cytotoxicity (ADCC) activity in a reporter assay. When tested in vivo using VSV-MACV in a Stat2-/- mouse model, three MAbs significantly lowered viral loads in the spleen. Our work provides valuable insights into epitopes targeted by neutralizing antibodies that could be potent targets for vaccines and therapeutics and shed light on the importance of effector functions in immunity against MACV.IMPORTANCE MACV infections are a significant public health concern and lead to high case fatality rates. No specific treatment or vaccine for MACV infections exist. However, cases of Junin virus infection, a related virus, can be treated with convalescent-phase serum. This indicates that a MAb-based therapy for MACV could be effective. Here, we describe several MAbs that neutralize MACV and could be used for this purpose.
Keywords: MACV; Machupo virus; arenavirus; monoclonal antibody.
Copyright © 2020 American Society for Microbiology.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

