Randomised, multicentre prospective trial of transarterial chemoembolisation (TACE) plus sorafenib as compared with TACE alone in patients with hepatocellular carcinoma: TACTICS trial
- PMID: 31801872
- PMCID: PMC7398460
- DOI: 10.1136/gutjnl-2019-318934
Randomised, multicentre prospective trial of transarterial chemoembolisation (TACE) plus sorafenib as compared with TACE alone in patients with hepatocellular carcinoma: TACTICS trial
Abstract
Objective: This trial compared the efficacy and safety of transarterial chemoembolisation (TACE) plus sorafenib with TACE alone using a newly established TACE-specific endpoint and pre-treatment of sorafenib before initial TACE.
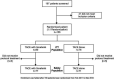
Design: Patients with unresectable hepatocellular carcinoma (HCC) were randomised to TACE plus sorafenib (n=80) or TACE alone (n=76). Patients in the combination group received sorafenib 400 mg once daily for 2-3 weeks before TACE, followed by 800 mg once daily during on-demand conventional TACE sessions until time to untreatable (unTACEable) progression (TTUP), defined as untreatable tumour progression, transient deterioration to Child-Pugh C or appearance of vascular invasion/extrahepatic spread. Co-primary endpoints were progression-free survival (PFS), which is not a conventional one but defined as TTUP, or time to any cause of death plus overall survival (OS). Multiplicity was adjusted by gatekeeping hierarchical testing.
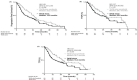
Results: Median PFS was significantly longer in the TACE plus sorafenib than in the TACE alone group (25.2 vs 13.5 months; p=0.006). OS was not analysed because only 73.6% of OS events were reached. Median TTUP (26.7 vs 20.6 months; p=0.02) was also significantly longer in the TACE plus sorafenib group. OS at 1 year and 2 years in TACE plus sorafenib group and TACE alone group were 96.2% and 82.7% and 77.2% and 64.6%, respectively. There were no unexpected toxicities.
Conclusion: TACE plus sorafenib significantly improved PFS over TACE alone in patients with unresectable HCC. Adverse events were consistent with those of previous TACE combination trials.
Trial registration number: NCT01217034.
Keywords: combination therapy with transarterial chemoembolisation and sorafenib; hepatocellular carcinoma; progression-free survival; sorafenib; transarterial chemoembolization.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: KM: Honoraria from Bayer, Eisai, MSD, Ajinomoto. Consulting or advisory role for Kowa, MSD, BMS, Bayer, Chugai, Taiho. Research funding from Chugai, Otuka, Takeda, Taiho, Sumitomo Dainippon, Daiichi Sankyo, MSD, Eisai, Bayer, Abbvie. IM: Honoraria from Novartis Pharma, Bayer Yakuhin, Bristol-Myers Squibb, Abbott Japan, Eisai, Taiho Pharmaceutical, Eli Lilly Japan, Daiichi-Sankyo, Yakult, Otsuka Pharmaceutical, Nobelpharma, EA Pharma, Teijin Pharma. Consulting or advisory role for Nano Carrier, Bayer Yakuhin, Eisai, Kyowa Hakko Kirin, Novartis Pharma, Shire, MSD, Bristol Myers Sqiibb, Eli Lilly Japan, Sumitomo Dainippon, Daiichi-Sankyo, Teijin Pharma, Takara Bio. Research funding from Bayer Yakuhin, Kyowa Hakko Kirin, Yakult, Taiho Pharmaceutical, Eli Lilly Japan, Ono Pharmaceutical, Eisai, AstraZeneca, Zeria Pharmaceutical, Chugai, Bristol Myers Sqiibb, Merck Serono, Kowa, Nano Carrier, ASLAN, Daiichi-Sankyo., Sumitomo Dainippon, Novartis Pharma, Baxalta, Boehringer Ingelheim, Takara Bio. Board membership: ASLAN, Chugai. IN: Honoraria from Bayer, Gilead, Abbvie, Otuka. IN: Research funding from Abbvie GK. HK: Honoraria from MSD, Abbvie, Bristrol-Myers Squibb, Dainippon Sumitomo and Otsuka, Research funding from Gilead, Bristrol-Myers Squibb, MSD. IA: Honoraria from AbbVie GK, Bristol Myers Squibb, Gilead, Eisai. Research funding from AbbVie GK, Otsuka, MSD, Chugai, Eisai, Astellas, Takeda. KN: Research funding from Abbvie GK.Nakao K: Honoraria from Gilead. YO: Research funding from AstraZeneca, Asteras, Ajinomoto, AsahiKasei-Kurare Medical, Bayer Yakuhin, Bristol Myers Sqiibb, Chugai, Daiichi-Sankyo, Eisai, Kyowa Hakko Kirin, Kowa, Nihon Kayaku, MSD, Otsuka, Ono, Taiho, Tanabe-Mitsubishi, Takeda, Torii, Tsumura, Zeria. YK: Honoraria from Chugai, Eli Lilly, Taiho, Nippon Shinyaku. OT: Honoraria from Novartis, Taiho, Eli Lilly, Dainippon Sumitomo, Bayer, Yakult, FUJIFILM, AstraZeneca, Ono Pharmaceutical, EA Pharma, Nippon Chemiphar, Celgene, Chugai, Bristol Myers, Eisai, Pfizer, Teijin, Daiichi Sankyo, MSD, Shire, AbbVie, Takeda. Consulting or advisory role for Eli Lilly, Dainippon Sumitomo, Taiho, Zeria Pharmaceutical, Daiichi Sankyo, Bristol Myers. Research Funding from Eli Lilly, Eisai, Novartis, Yakult Honsha, Taiho, Kowa, Kyowa Hakko Kirin, Merck Serono, Ono Pharmaceutical, Bayer, Pfizer Japan, AstraZeneca, Dainippon Sumitomo, Chugai, Bristol Myers, Zeria Pharmaceutical. KN: Honoraria from Eisai. AY: Royalties from Sumitomo Bakelite. Research funding from Canon Medical Systems, Taiho Pharmaceutical, Eisai. Honoraria from Merit Medical Systems., Fuji Pharma, Terumo International Systems, Nippon Kayaku, Canon Medical Systems, Bristol Meyer Squibb, Sumitomo Bakelite, Bayer Pharmaceuticals, Boston Scientific Japan, Taiho Pharmaceutical, Guerbet Japan, Guerbet Asia Pacific.
Figures

Comment in
-
Changing TACTICS in intermediate HCC: TACE plus sorafenib.Gut. 2020 Aug;69(8):1374-1376. doi: 10.1136/gutjnl-2020-320692. Epub 2020 Mar 13. Gut. 2020. PMID: 32169908 Free PMC article. No abstract available.
-
Combination therapy of transcatheter arterial chemoembolization with axitinib for the treatment of inoperable hepatocellular carcinoma.Ann Transl Med. 2020 Aug;8(16):1039. doi: 10.21037/atm.2020.03.198. Ann Transl Med. 2020. PMID: 32953839 Free PMC article. No abstract available.
-
Transarterial chemoembolization (TACE) plus sorafenib: a real winning combination?Ann Transl Med. 2020 Dec;8(23):1616. doi: 10.21037/atm-20-4268. Ann Transl Med. 2020. PMID: 33437815 Free PMC article. No abstract available.
References
-
- European association for the study of the liver. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2018;69:182–236. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
