Frontal cortex neuron types categorically encode single decision variables
- PMID: 31801999
- PMCID: PMC10197198
- DOI: 10.1038/s41586-019-1816-9
Frontal cortex neuron types categorically encode single decision variables
Abstract
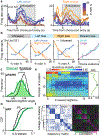
Individual neurons in many cortical regions have been found to encode specific, identifiable features of the environment or body that pertain to the function of the region1-3. However, in frontal cortex, which is involved in cognition, neural responses display baffling complexity, carrying seemingly disordered mixtures of sensory, motor and other task-related variables4-13. This complexity has led to the suggestion that representations in individual frontal neurons are randomly mixed and can only be understood at the neural population level14,15. Here we show that neural activity in rat orbitofrontal cortex (OFC) is instead highly structured: single neuron activity co-varies with individual variables in computational models that explain choice behaviour. To characterize neural responses across a large behavioural space, we trained rats on a behavioural task that combines perceptual and value-guided decisions. An unbiased, model-free clustering analysis identified distinct groups of OFC neurons, each with a particular response profile in task-variable space. Applying a simple model of choice behaviour to these categorical response profiles revealed that each profile quantitatively corresponds to a specific decision variable, such as decision confidence. Additionally, we demonstrate that a connectivity-defined cell type, orbitofrontal neurons projecting to the striatum, carries a selective and temporally sustained representation of a single decision variable: integrated value. We propose that neurons in frontal cortex, as in other cortical regions, form a sparse and overcomplete representation of features relevant to the region's function, and that they distribute this information selectively to downstream regions to support behaviour.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

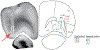





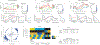






References
-
- Bruce C, Desimone R & Gross CG Visual properties of neurons in a polysensory area in superior temporal sulcus of the macaque. J. Neurophysiol 46, 369–84 (1981). - PubMed
-
- O’Keefe J & Dostrovsky J The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Res. 34, 171–5 (1971). - PubMed
-
- Feierstein CE, Quirk MC, Uchida N, Sosulski DL & Mainen ZF Representation of spatial goals in rat orbitofrontal cortex. Neuron 51, 495–507 (2006). - PubMed
References (Methods)
-
- Kepecs A, Uchida N, Zariwala HA & Mainen ZF Neural correlates, computation and behavioural impact of decision confidence. Nature 455, 227–31 (2008). - PubMed
-
- Li SJ, Vaughan A, Sturgill JF & Kepecs A A Viral Receptor Complementation Strategy to Overcome CAV-2 Tropism for Efficient Retrograde Targeting of Neurons. Neuron 98, 905–917.e5 (2018). - PubMed
-
- Soudais C, Laplace-Builhe C, K K & Kremer E. Preferential transduction of neurons by canine adenovirus vectors and their efficient retrograde transport in vivo. FASEB J. 15:, 2283–5 (2001). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

