The molecular landscape of ETMR at diagnosis and relapse
- PMID: 31802000
- PMCID: PMC6908757
- DOI: 10.1038/s41586-019-1815-x
The molecular landscape of ETMR at diagnosis and relapse
Abstract
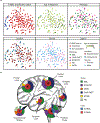
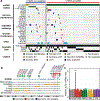
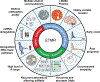
Embryonal tumours with multilayered rosettes (ETMRs) are aggressive paediatric embryonal brain tumours with a universally poor prognosis1. Here we collected 193 primary ETMRs and 23 matched relapse samples to investigate the genomic landscape of this distinct tumour type. We found that patients with tumours in which the proposed driver C19MC2-4 was not amplified frequently had germline mutations in DICER1 or other microRNA-related aberrations such as somatic amplification of miR-17-92 (also known as MIR17HG). Whole-genome sequencing revealed that tumours had an overall low recurrence of single-nucleotide variants (SNVs), but showed prevalent genomic instability caused by widespread occurrence of R-loop structures. We show that R-loop-associated chromosomal instability can be induced by the loss of DICER1 function. Comparison of primary tumours and matched relapse samples showed a strong conservation of structural variants, but low conservation of SNVs. Moreover, many newly acquired SNVs are associated with a mutational signature related to cisplatin treatment. Finally, we show that targeting R-loops with topoisomerase and PARP inhibitors might be an effective treatment strategy for this deadly disease.
Conflict of interest statement
Declaration of Interests
The authors declare no competing interests
Figures









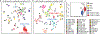



References
-
- Eberhart CG, Brat DJ, Cohen KJ & Burger PC Pediatric neuroblastic brain tumors containing abundant neuropil and true rosettes. Pediatr Dev Pathol 3, 346–352 (2000). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

