Regulation of α-synuclein by chaperones in mammalian cells
- PMID: 31802003
- PMCID: PMC6930850
- DOI: 10.1038/s41586-019-1808-9
Regulation of α-synuclein by chaperones in mammalian cells
Abstract
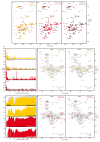
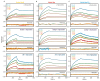
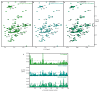
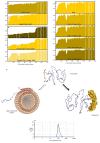
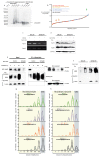
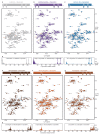
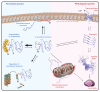
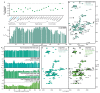
Neurodegeneration in patients with Parkinson's disease is correlated with the occurrence of Lewy bodies-intracellular inclusions that contain aggregates of the intrinsically disordered protein α-synuclein1. The aggregation propensity of α-synuclein in cells is modulated by specific factors that include post-translational modifications2,3, Abelson-kinase-mediated phosphorylation4,5 and interactions with intracellular machineries such as molecular chaperones, although the underlying mechanisms are unclear6-8. Here we systematically characterize the interaction of molecular chaperones with α-synuclein in vitro as well as in cells at the atomic level. We find that six highly divergent molecular chaperones commonly recognize a canonical motif in α-synuclein, consisting of the N terminus and a segment around Tyr39, and hinder the aggregation of α-synuclein. NMR experiments9 in cells show that the same transient interaction pattern is preserved inside living mammalian cells. Specific inhibition of the interactions between α-synuclein and the chaperone HSC70 and members of the HSP90 family, including HSP90β, results in transient membrane binding and triggers a remarkable re-localization of α-synuclein to the mitochondria and concomitant formation of aggregates. Phosphorylation of α-synuclein at Tyr39 directly impairs the interaction of α-synuclein with chaperones, thus providing a functional explanation for the role of Abelson kinase in Parkinson's disease. Our results establish a master regulatory mechanism of α-synuclein function and aggregation in mammalian cells, extending the functional repertoire of molecular chaperones and highlighting new perspectives for therapeutic interventions for Parkinson's disease.
Conflict of interest statement
The authors declare no competing financial interests.
Figures





References
-
- Goedert M, Spillantini MG, Del Tredici K, Braak H. 100 years of Lewy pathology. Nat Rev Neurol. 2013;9:13–24. - PubMed
-
- Barnham KJ, Masters CL, Bush AI. Neurodegenerative diseases and oxidative stress. Nat Rev Drug Discov. 2004;3:205–214. - PubMed
-
- Hantschel O, Superti-Furga G. Regulation of the c-Abl and Bcr-Abl tyrosine kinases. Nat Rev Mol Cell Biol. 2004;5:33–44. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

