Population Pharmacokinetic Modeling of Acetaminophen Protein Adducts in Adults and Children
- PMID: 31802503
- PMCID: PMC7643159
- DOI: 10.1002/jcph.1555
Population Pharmacokinetic Modeling of Acetaminophen Protein Adducts in Adults and Children
Abstract
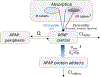
Acetaminophen protein adducts (adducts) are a well-established biomarker to diagnose acetaminophen toxicity. To date, the quantitative relationship between acetaminophen exposure, which drives adduct formation, and adduct exposure remains to be established. Our study characterized the adduct formation and disposition in adults using the approach of population parent-metabolite modeling. It demonstrated formation-limited pharmacokinetics (PK) for adducts in healthy subjects. This finding expands the existing knowledge on adduct PK that showed an apparent long elimination half-life. We then allometrically scaled the adduct PK model to children, simulated the adduct profiles, and compared these simulated profiles with those observed in an independent cohort of children. The scaled model significantly overpredicted the adduct concentrations in children early on in treatment and underpredicted concentrations following repeated acetaminophen doses. These results suggest that children demonstrate different adduct PK behavior from that of adults, most likely because of increased reactive metabolite detoxification in children. In summary, we described the first PK model linking acetaminophen and acetaminophen protein adduct concentrations, which provides a semimechanistic understanding of varying profiles of adduct exposure in adults and children.
Keywords: acetaminophen protein adducts; biomarkers; hepatotoxicity; parent-metabolite model; pediatrics.
© 2019, The American College of Clinical Pharmacology.
Conflict of interest statement
Conflict of Interest
Dr. Laura James is a part owner of Acetaminophen Toxicity Diagnostics (ATD), LLC. ATD is developing a rapid assay for the measurement of acetaminophen protein adducts in human blood samples.
Figures


Similar articles
-
Acetaminophen Protein Adducts in Hospitalized Children Receiving Multiple Doses of Acetaminophen.J Clin Pharmacol. 2019 Oct;59(10):1291-1299. doi: 10.1002/jcph.1442. Epub 2019 May 17. J Clin Pharmacol. 2019. PMID: 31099052 Free PMC article.
-
Early acetaminophen-protein adducts predict hepatotoxicity following overdose (ATOM-5).J Hepatol. 2020 Mar;72(3):450-462. doi: 10.1016/j.jhep.2019.10.030. Epub 2019 Nov 22. J Hepatol. 2020. PMID: 31760072 Clinical Trial.
-
Prolonged Acetaminophen-Protein Adduct Elimination During Renal Failure, Lack of Adduct Removal by Hemodiafiltration, and Urinary Adduct Concentrations After Acetaminophen Overdose.J Med Toxicol. 2015 Jun;11(2):169-78. doi: 10.1007/s13181-014-0431-2. J Med Toxicol. 2015. PMID: 25288219 Free PMC article.
-
Covalent binding of xenobiotics to specific proteins in the liver.Drug Metab Rev. 1997 Feb-May;29(1-2):39-57. doi: 10.3109/03602539709037572. Drug Metab Rev. 1997. PMID: 9187510 Review.
-
Monitoring drug-protein interaction.Clin Chim Acta. 2006 Mar;365(1-2):9-29. doi: 10.1016/j.cca.2005.08.021. Epub 2005 Sep 30. Clin Chim Acta. 2006. PMID: 16199025 Review.
Cited by
-
Population pharmacokinetic of paracetamol and atorvastatin with co-administration of semaglutide.Pharmacol Res Perspect. 2022 Aug;10(4):e00962. doi: 10.1002/prp2.962. Pharmacol Res Perspect. 2022. PMID: 35799471 Free PMC article.
-
[Advancements in the diagnosis and treatment of pediatric acute liver failure].Zhongguo Dang Dai Er Ke Za Zhi. 2024 Feb 15;26(2):194-200. doi: 10.7499/j.issn.1008-8830.2309015. Zhongguo Dang Dai Er Ke Za Zhi. 2024. PMID: 38436319 Free PMC article. Review. Chinese.
References
-
- Davern TJ. Indeterminate acute liver failure: a riddle wrapped in a mystery inside an enigma. Hepatology. 2006;44(3):765–768.
-
- Qiu Y, Benet LZ, Burlingame AL. Identification of the hepatic protein targets of reactive metabolites of acetaminophen in vivo in mice using two-dimensional gel electrophoresis and mass spectrometry. J Biol Chem. 1998;273(28):17940–17953. - PubMed
-
- James LP, Mayeux PR, Hinson JA. Acetaminophen-induced hepatotoxicity. Drug Metab Dispos. 2003;31(12):1499–1506. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

