Comparative dental anatomy in newborn primates: Cusp mineralization
- PMID: 31802627
- PMCID: PMC7269855
- DOI: 10.1002/ar.24326
Comparative dental anatomy in newborn primates: Cusp mineralization
Abstract
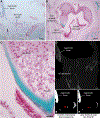
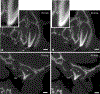
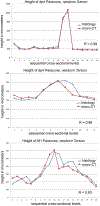
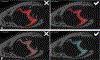
Previous descriptive work on deciduous dentition of primates has focused disproportionately on great apes and humans. To address this bias in the literature, we studied 131 subadult nonhominoid specimens (including 110 newborns) describing deciduous tooth morphology and assessing maximum hydroxyapatite density (MHD). All specimens were CT scanned at 70 kVp and reconstructed at 20.5-39 μm voxels. Grayscale intensity from scans was converted to hydroxyapatite (HA) density (mg HA/cm3 ) using a linear conversion of grayscale values to calibration standards of known HA density (R2 = .99). Using Amira software, mineralized dental tissues were captured by segmenting the tooth cusps first and then capturing the remainder of the teeth at descending thresholds of gray levels. We assessed the relationship of MHD of selected teeth to cranial length using Pearson correlation coefficients. In monkeys, anterior teeth are more mineralized than postcanine teeth. In tarsiers and most lemurs and lorises, postcanine teeth are the most highly mineralized. This suggests that monkeys have a more prolonged process of dental mineralization that begins with incisors and canines, while mineralization of postcanine teeth is delayed. This may in part be a result of relatively late weaning in most anthropoid primates. Results also reveal that in lemurs and lorises, MHD of the mandibular first permanent molar (M1 ) negatively correlates with cranial length. In contrast, the MHD of M1 positively correlates with cranial length in monkeys. This supports the hypothesis that natural selection acts independently on dental growth as opposed to mineralization and indicates clear phylogenetic differences among primates.
Keywords: catarrhine; deciduous; dentition; platyrrhine.
© 2019 American Association for Anatomy.
Figures


















References
-
- Ahrens H. (1913). Die Entwicklung der menschlichen Zähne. Anatomische Hefte, 48, 169–257.
-
- Anemone RL, Mooney MP, & Siegel MI (1996). Longitudinal study of dental development in chimpanzees of known chronological age: Implications for understanding age at death of Plio-Pleistocene hominids. American Journal of Physical Anthropology, 99, 119–133. - PubMed
-
- Anemone RL, Skinner MM, & Dirks W. (2012). Are there two distinct types of hypocone in Eocene primates? The ‘pseudohypocone’ of notharctines revisited. Paleontologia Electronica, 15, 26A.
-
- Avery JK (2002). Oral development and histology (3rd ed.). New York, NY: Thieme Medical Publishers.
-
- Benefit BR (1994). Phylogenetic, paleodemographic, and taphonomic implications of Victoriapithecus deciduous teeth from Maboko, Kenya. American Journal of Physical Anthropology, 95, 277–331. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

