Two-drug antiretroviral regimens: an assessment of virologic response and durability among treatment-experienced persons living with HIV in the OPERA® Observational Database
- PMID: 31802641
- PMCID: PMC6893210
- DOI: 10.1002/jia2.25418
Two-drug antiretroviral regimens: an assessment of virologic response and durability among treatment-experienced persons living with HIV in the OPERA® Observational Database
Abstract
Introduction: Two-drug regimens (2-DR) have the potential to be a viable solution to the challenges of treatment complexity, cost, adverse effects and contraindications. We sought to describe the real-world use and effectiveness of 2-DR among persons living with HIV (PLHIV) in the United States.
Methods: We analysed data for 10,190 treatment-experienced patients from the OPERA® Observational Database initiating a new 2-DR or three-drug regimen (3-DR) between 1 January 2010 and 30 June 2016. Multivariate Cox Proportional Hazards models were used to estimate the association among 2-DR or 3-DR initiation and virologic suppression (viral load (VL) <50 copies/mL), virologic failure (2 VLs > 200 copies/mL or 1 VL > 200 copies/mL + discontinuation) or regimen discontinuation.
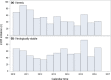
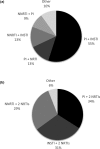
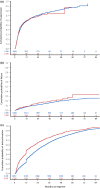
Results: Patients initiating a 2-DR (n = 1337, 13%) were older, and more likely to have a lower CD4 count, a history of AIDS and comorbid conditions than patients initiating a 3-DR. There was no difference between groups in time to virologic suppression (aHR: 1.00 (95% CI: 0.88, 1.13)) among viraemic patients (baseline VL ≥ 50 copies/mL, n = 4180), or time to virologic failure (aHR: 1.15 (95% CI: 0.90, 1.48)) among virologically stable patients (baseline VL < 50 copies/mL, n = 6010). However, time to discontinuation was shorter following 2-DR than 3-DR initiation (aHR: 1.51 (95% CI: 1.41, 1.61)).
Conclusions: In this large cohort of treatment-experienced patients, 2-DR prescriptions were common and more frequent among patients with significant comorbidity. Virologic response was similar, but duration of use was shorter with a 2-DR than a 3-DR, suggesting that 2-DRs may be a virologically effective treatment strategy for treatment-experienced PLHIV with existing comorbidities.
Keywords: ART-experience; HIV; antiretroviral therapy-experience; cohort; human immunodeficiency virus; regimens; two-drug.
© 2019 The Authors. Journal of the International AIDS Society published by John Wiley & Sons Ltd on behalf of the International AIDS Society.
Figures
References
-
- Hall AM, Hendry BM, Nitsch D, Connolly JO. Tenofovir‐associated kidney toxicity in HIV‐infected patients: a review of the evidence. Am J Kidney Dis. 2011;57(5):773–80. - PubMed
-
- Panel on Antiretroviral Guidelines for Adults and Adolescent . Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents Living with HIV Department of Health and Human Services. 2018. [cited 2018 Aug 14]. Available from: http://aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

