Association between Gut Microbiome Composition and Rotavirus Vaccine Response among Nicaraguan Infants
- PMID: 31802728
- PMCID: PMC6947780
- DOI: 10.4269/ajtmh.19-0355
Association between Gut Microbiome Composition and Rotavirus Vaccine Response among Nicaraguan Infants
Abstract
Rotavirus is the leading cause of childhood deaths due to diarrhea. Although existing oral rotavirus vaccines are highly efficacious in high-income countries, these vaccines have been demonstrated to have decreased efficacy in low- and middle-income countries. A possible explanation for decreased efficacy is the impact of gut microbiota on the enteric immune system's response to vaccination. We analyzed the gut microbiome of 50 children enrolled in a prospective study evaluating response to oral pentavalent rotavirus vaccination (RV5) to assess associations between relative abundance of bacterial taxa and seroconversion following vaccination. Stool samples were taken before the first RV5 dose, and microbiome composition characterized using 16S rRNA amplicon sequencing and Quantitative Insights Into Microbial Ecology software. Relative abundance of bacterial taxa between seroconverters following the first RV5 dose, those with ≥ 4-fold increase in rotavirus-specific IgA titers, and nonseroconverters were compared using the Wilcoxon-Mann-Whitney test. We identified no significant differences in microbiome composition between infants who did and did not respond to vaccination. Infants who responded to vaccination tended to have higher abundance of Proteobacteria and Eggerthella, whereas those who did not respond had higher abundance of Fusobacteria and Enterobacteriaceae; however, these differences were not statistically significant following a multiple comparison correction. This study suggests a limited impact of gut microbial taxa on response to oral rotavirus vaccination among infants; however, additional research is needed to improve our understanding of the impact of gut microbiome on vaccine response, toward a goal of improving vaccine efficacy and rotavirus prevention.
Figures
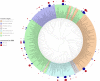
References
-
- Liu L, et al. Child Health Epidemiology Reference Group of WHO, Unicef , 2012. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet 379: 2151–2161. - PubMed
-
- Kotloff KL, et al. 2013. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the global enteric multicenter study, GEMS): a prospective, case-control study. Lancet 382: 209–222. - PubMed
-
- Vesikari T, et al. 2006. Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. N Engl J Med 354: 23–33. - PubMed
-
- Armah GE, et al. 2010. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in sub-Saharan Africa: a randomised, double-blind, placebo-controlled trial. Lancet 376: 606–614. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

