CTGF Loaded Electrospun Dual Porous Core-Shell Membrane For Diabetic Wound Healing
- PMID: 31802870
- PMCID: PMC6827515
- DOI: 10.2147/IJN.S224047
CTGF Loaded Electrospun Dual Porous Core-Shell Membrane For Diabetic Wound Healing
Abstract
Purpose: Impairment of wound healing is a major issue in type-2 diabetes that often causes chronic infections, eventually leading to limb and/or organ amputation. Connective tissue growth factor (CTGF) is a signaling molecule with several roles in tissue repair and regeneration including promoting cell adhesion, cell migration, cell proliferation and angiogenesis. Incorporation of CTGF in a biodegradable core-shell fiber to facilitate its sustained release is a novel approach to promote angiogenesis, cell migration and facilitate wound healing. In this paper, we report the development of CTGF encapsulated electrospun dual porous PLA-PVA core-shell fiber based membranes for diabetic wound healing applications.
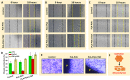
Methods: The membranes were fabricated by a core-shell electrospinning technique. CTGF was entrapped within the PVA core which was coated by a thin layer of PLA. The developed membranes were characterized by techniques such as Scanning Electron Microscopy (SEM), Fourier Transform Infrared Spectroscopy (FTIR) and X-Ray Diffraction (XRD) analysis. In vitro cell culture studies using fibroblasts, keratinocytes and endothelial cells were performed to understand the effect of CTGF loaded membranes on cell proliferation, cell viability and cell migration. A chicken chorioallantoic membrane (CAM) assay was performed to determine the angiogenic potential of the membranes.
Results: Results showed that the developed membranes were highly porous in morphology with secondary pore formation on the surface of individual fibers. In vitro cell culture studies demonstrated that CTGF loaded core-shell membranes improved cell viability, cell proliferation and cell migration. A sustained release of CTGF from the core-shell fibers was observed for an extended time period. Moreover, the CAM assay showed that core-shell membranes incorporated with CTGF can enhance angiogenesis.
Conclusion: Owing to the excellent cell proliferation, migration and angiogenic potential of CTGF loaded core-shell PLA-PVA fibrous membranes, they can be used as an excellent wound dressing membrane for treating diabetic wounds and other chronic ulcers.
Keywords: CTGF; PLA; PVA; diabetic wound; electrospinning.
© 2019 Augustine et al.
Conflict of interest statement
The authors declare no competing interests in this work.
Figures





References
-
- Cunha M, Queirós P, Cardoso D, Santos E, Rodrigues M, Apóstolo J. The effectiveness of cleansing solutions for wound treatment: a systematic review. JBI Database System Rev Implement Rep. 2014;12(10):121–151. doi:10.11124/jbisrir-2014-1746 - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

