Mechanisms of Immune Tolerance in Liver Transplantation-Crosstalk Between Alloreactive T Cells and Liver Cells With Therapeutic Prospects
- PMID: 31803188
- PMCID: PMC6877506
- DOI: 10.3389/fimmu.2019.02667
Mechanisms of Immune Tolerance in Liver Transplantation-Crosstalk Between Alloreactive T Cells and Liver Cells With Therapeutic Prospects
Abstract
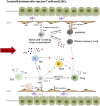
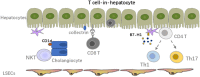
Liver transplantation (LTx) is currently the most powerful treatment for end-stage liver disease. Although liver allograft is more tolerogenic compared to other solid organs, the majority of LTx recipients still require long-term immune suppression (IS) to control the undesired alloimmune responses, which can lead to severe side effects. Thus, understanding the mechanism of liver transplant tolerance and crosstalk between immune cells, especially alloreactive T cells and liver cells, can shed light on more specific tolerance induction strategies for future clinical translation. In this review, we focus on alloreactive T cell mediated immune responses and their crosstalk with liver sinusoidal endothelial cells (LSECs), hepatocytes, hepatic stellate cells (HSCs), and cholangiocytes in transplant setting. Liver cells mainly serve as antigen presenting cells (APCs) to T cells, but with low expression of co-stimulatory molecules. Crosstalk between them largely depends on the different expression of adhesion molecules and chemokine receptors. Inflammatory cytokines secreted by immune cells further elaborate this crosstalk and regulate the fate of naïve T cells differentiation within the liver graft. On the other hand, regulatory T cells (Tregs) play an essential role in inducing and keeping immune tolerance in LTx. Tregs based adoptive cell therapy provides an excellent therapeutic option for clinical transplant tolerance induction. However, many questions regarding cell therapy still need to be solved. Here we also address the current clinical trials of adoptive Tregs therapy and other tolerance induction strategies in LTx, together with future challenges for clinical translation from bench to bedside.
Keywords: alloreactive T cells; crosstalk; liver cells; liver transplantation; tolerance induction.
Copyright © 2019 Lei, Reinke, Volk, Lv and Wu.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

