B Cell Tetherin: A Flow Cytometric Cell-Specific Assay for Response to Type I Interferon Predicts Clinical Features and Flares in Systemic Lupus Erythematosus
- PMID: 31804007
- PMCID: PMC8653884
- DOI: 10.1002/art.41187
B Cell Tetherin: A Flow Cytometric Cell-Specific Assay for Response to Type I Interferon Predicts Clinical Features and Flares in Systemic Lupus Erythematosus
Abstract
Objective: Type I interferon (IFN) responses are broadly associated with autoimmune diseases, including systemic lupus erythematosus (SLE). Given the cardinal role of autoantibodies in SLE, this study was undertaken to investigate whether the findings of a B cell-specific IFN assay correlate with SLE activity.
Methods: B cells and peripheral blood mononuclear cells (PBMCs) were stimulated with type I IFN and type II IFN. Gene expression was analyzed, and the expression of pathway-related membrane proteins was determined. A flow cytometry assay for tetherin (CD317), an IFN-induced protein ubiquitously expressed on leukocytes, was validated in vitro and then clinically against SLE diagnosis, plasmablast expansion, and the British Isles Lupus Assessment Group (BILAG) 2004 score in a discovery cohort (n = 156 SLE patients, 30 rheumatoid arthritis [RA] patients, and 25 healthy controls). A second, longitudinal validation cohort of 80 SLE patients was also evaluated for flare prediction.
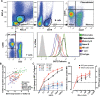
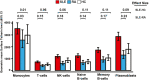
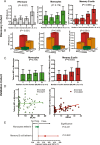
Results: In vitro, a close cell-specific and dose-response relationship between type I IFN-responsive genes and cell surface tetherin was observed in all immune cell subsets. Tetherin expression on multiple cell subsets was selectively responsive to stimulation with type I IFN compared to types II and III IFNs. In patient samples from the discovery cohort, memory B cell tetherin showed the strongest associations with diagnosis (SLE:healthy control effect size 0.11 [P = 0.003]; SLE:RA effect size 0.17 [P < 0.001]), plasmablast numbers in rituximab-treated patients (R = 0.38, P = 0.047), and BILAG 2004. These associations were equivalent to or stronger than those for IFN score or monocyte tetherin. Memory B cell tetherin was found to be predictive of future clinical flares in the validation cohort (hazard ratio 2.29 [95% confidence interval 1.01-4.64]; P = 0.022).
Conclusion: Our findings indicate that memory B cell surface tetherin, a B cell-specific IFN assay, is associated with SLE diagnosis and disease activity, and predicts flares better than tetherin on other cell subsets or whole blood assays, as determined in an independent validation cohort.
© 2019 The Authors. Arthritis & Rheumatology published by Wiley Periodicals, Inc. on behalf of American College of Rheumatology.
Figures
References
-
- Rönnblom L, Eloranta ML. The interferon signature in autoimmune diseases. Curr Opin Rheumatol 2013;25:248–53. - PubMed
-
- Vidal‐Laliena M, Romero X, March S, Requena V, Petriz J, Engel P. Characterization of antibodies submitted to the B cell section of the 8th Human Leukocyte Differentiation Antigens Workshop by flow cytometry and immunohistochemistry. Cell Immunol 2005;236:6–16. - PubMed
-
- Evans EJ, Hene L, Sparks LM, Dong T, Retiere C, Fennelly JA, et al. The T cell surface—how well do we know it? Immunity 2003;19:213–23. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

