Unpacking the aggregation-oligomerization-fibrillization process of naturally-occurring hIAPP amyloid oligomers isolated directly from sera of children with obesity or diabetes mellitus
- PMID: 31804529
- PMCID: PMC6895187
- DOI: 10.1038/s41598-019-54570-8
Unpacking the aggregation-oligomerization-fibrillization process of naturally-occurring hIAPP amyloid oligomers isolated directly from sera of children with obesity or diabetes mellitus
Abstract
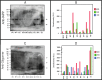
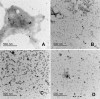
The formation of amyloid oligomers and fibrils of the human islet amyloid polypeptide (hIAPP) has been linked with β- cell failure and death which causes the onset, progression, and comorbidities of diabetes. We begin to unpack the aggregation-oligomerization-fibrillization process of these oligomers taken from sera of pediatric patients. The naturally occurring or real hIAPP (not synthetic) amyloid oligomers (RIAO) were successfully isolated, we demonstrated the presence of homo (dodecamers, hexamers, and trimers) and hetero-RIAO, as well as several biophysical characterizations which allow us to learn from the real phenomenon taking place. We found that the aggregation/oligomerization process is active in the sera and showed that it happens very fast. The RIAO can form fibers and react with anti-hIAPP and anti-amyloid oligomers antibodies. Our results opens the epistemic horizon and reveal real differences between the four groups (Controls vs obesity, T1DM or T2DM) accelerating the process of understanding and discovering novel and more efficient prevention, diagnostic, transmission and therapeutic pathways.
Conflict of interest statement
The authors declare no competing interests.
Figures






References
-
- Sontag, E. M., Samant, R. S. & Frydman, J. Mechanisms and Functions of Spatial Protein Quality Control., 10.1146/annurev-biochem (2017). - PubMed
-
- Knowles, T. P., Vendruscolo, M. & Dobson, C. M. The amyloid state and its association with protein misfolding diseases. Nat.Rev.Mol.Cell Biol. 15, 384–396 (2014). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

