Effect of Thoracoscopic Talc Poudrage vs Talc Slurry via Chest Tube on Pleurodesis Failure Rate Among Patients With Malignant Pleural Effusions: A Randomized Clinical Trial
- PMID: 31804680
- PMCID: PMC6990658
- DOI: 10.1001/jama.2019.19997
Effect of Thoracoscopic Talc Poudrage vs Talc Slurry via Chest Tube on Pleurodesis Failure Rate Among Patients With Malignant Pleural Effusions: A Randomized Clinical Trial
Abstract
Importance: Malignant pleural effusion (MPE) is challenging to manage. Talc pleurodesis is a common and effective treatment. There are no reliable data, however, regarding the optimal method for talc delivery, leading to differences in practice and recommendations.
Objective: To test the hypothesis that administration of talc poudrage during thoracoscopy with local anesthesia is more effective than talc slurry delivered via chest tube in successfully inducing pleurodesis.
Design, setting, and participants: Open-label, randomized clinical trial conducted at 17 UK hospitals. A total of 330 participants were enrolled from August 2012 to April 2018 and followed up until October 2018. Patients were eligible if they were older than 18 years, had a confirmed diagnosis of MPE, and could undergo thoracoscopy with local anesthesia. Patients were excluded if they required a thoracoscopy for diagnostic purposes or had evidence of nonexpandable lung.
Interventions: Patients randomized to the talc poudrage group (n = 166) received 4 g of talc poudrage during thoracoscopy while under moderate sedation, while patients randomized to the control group (n = 164) underwent bedside chest tube insertion with local anesthesia followed by administration of 4 g of sterile talc slurry.
Main outcomes and measures: The primary outcome was pleurodesis failure up to 90 days after randomization. Secondary outcomes included pleurodesis failure at 30 and 180 days; time to pleurodesis failure; number of nights spent in the hospital over 90 days; patient-reported thoracic pain and dyspnea at 7, 30, 90, and 180 days; health-related quality of life at 30, 90, and 180 days; all-cause mortality; and percentage of opacification on chest radiograph at drain removal and at 30, 90, and 180 days.
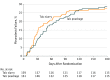
Results: Among 330 patients who were randomized (mean age, 68 years; 181 [55%] women), 320 (97%) were included in the primary outcome analysis. At 90 days, the pleurodesis failure rate was 36 of 161 patients (22%) in the talc poudrage group and 38 of 159 (24%) in the talc slurry group (adjusted odds ratio, 0.91 [95% CI, 0.54-1.55]; P = .74; difference, -1.8% [95% CI, -10.7% to 7.2%]). No statistically significant differences were noted in any of the 24 prespecified secondary outcomes.
Conclusions and relevance: Among patients with malignant pleural effusion, thoracoscopic talc poudrage, compared with talc slurry delivered via chest tube, resulted in no significant difference in the rate of pleurodesis failure at 90 days. However, the study may have been underpowered to detect small but potentially important differences.
Trial registration: ISRCTN Identifier: ISRCTN47845793.
Conflict of interest statement
Figures

Comment in
-
Delivery of Talc for Pleurodesis of Malignant Pleural Effusions.JAMA. 2020 May 12;323(18):1855. doi: 10.1001/jama.2020.3265. JAMA. 2020. PMID: 32396177 No abstract available.
-
Pleural Disease Management: Manometry-guided Thoracentesis, Optimal Drainage Regimen of Indwelling Pleural Catheters, and Talc Poudrage versus Slurry for Malignant Pleural Effusion.Am J Respir Crit Care Med. 2020 Aug 1;202(3):448-450. doi: 10.1164/rccm.202003-0599RR. Am J Respir Crit Care Med. 2020. PMID: 32421351 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

