Trends in mesenchymal stem cell clinical trials 2004-2018: Is efficacy optimal in a narrow dose range?
- PMID: 31804767
- PMCID: PMC6954709
- DOI: 10.1002/sctm.19-0202
Trends in mesenchymal stem cell clinical trials 2004-2018: Is efficacy optimal in a narrow dose range?
Abstract
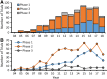
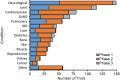
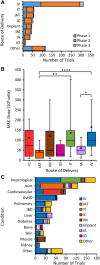
The number of clinical trials using mesenchymal stem cells (MSCs) has increased since 2008, but this trend slowed in the past several years and dropped precipitously in 2018. Previous reports have analyzed MSC clinical trials by disease, phase, cell source, country of origin, and trial initiation date, all of which can be downloaded directly from ClinicalTrials.gov. We have extended analyses to a larger group of 914 MSC trials reported through 2018. To search for potential factors that may influence the design of new trials, we extracted data on routes of administration and dosing from individual ClinicalTrials.gov records as this information cannot be downloaded directly from the database. Intravenous (IV) injection is the most common, least invasive and most reproducible method, accounting for 43% of all trials. The median dose for IV delivery is 100 million MSCs/patient/dose. Analysis of all trials using IV injection that reported positive outcomes indicated minimal effective doses (MEDs) ranging from 70 to 190 million MSCs/patient/dose in 14/16 trials with the other two trials administering much higher doses of at least 900 million cells. Dose-response data showing differential efficacy for improved outcomes were reported in only four trials, which indicated a narrower MED range of 100-150 million MSCs/patient with lower and higher IV doses being less effective. The results suggest that it may be critical to determine MEDs in early trials before proceeding with large clinical trials.
Keywords: ClinicalTrials.gov; dose; intravenous; mesenchymal stromal cell.
© 2020 The Authors. Stem Cells Translational Medicine published by Wiley Periodicals, Inc. on behalf of AlphaMed Press.
Conflict of interest statement
M.G. declared equity in CytoStormRx LLC but there is no conflict of interest. The other authors indicated no potential conflicts of interest.
Figures


References
-
- Oliveri RS, Bello S, Biering‐Sorensen F. Mesenchymal stem cells improve locomotor recovery in traumatic spinal cord injury: systematic review with meta‐analyses of rat models. Neurobiol Dis. 2014;62:338‐353. - PubMed
-
- Walter J, Ware LB, Matthay MA. Mesenchymal stem cells: mechanisms of potential therapeutic benefit in ARDS and sepsis. Lancet Respir Med. 2014;2:1016‐1026. - PubMed
-
- Yoo J, Kim HS, Hwang DY. Stem cells as promising therapeutic options for neurological disorders. J Cell Biochem. 2013;114:743‐753. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

