Phase III Trial of Adjuvant Capecitabine After Standard Neo-/Adjuvant Chemotherapy in Patients With Early Triple-Negative Breast Cancer (GEICAM/2003-11_CIBOMA/2004-01)
- PMID: 31804894
- PMCID: PMC6968797
- DOI: 10.1200/JCO.19.00904
Phase III Trial of Adjuvant Capecitabine After Standard Neo-/Adjuvant Chemotherapy in Patients With Early Triple-Negative Breast Cancer (GEICAM/2003-11_CIBOMA/2004-01)
Erratum in
-
Errata.J Clin Oncol. 2020 Mar 10;38(8):847. doi: 10.1200/JCO.20.00164. J Clin Oncol. 2020. PMID: 32135071 Free PMC article. No abstract available.
Abstract
Purpose: Operable triple-negative breast cancers (TNBCs) have a higher risk of relapse than non-TNBCs with standard therapy. The GEICAM/2003-11_CIBOMA/2004-01 trial explored extended adjuvant capecitabine after completion of standard chemotherapy in patients with early TNBC.
Patients and methods: Eligible patients were those with operable, node-positive-or node negative with tumor 1 cm or greater-TNBC, with prior anthracycline- and/or taxane-containing chemotherapy. After central confirmation of TNBC status by immunohistochemistry, patients were randomly assigned to either capecitabine or observation. Stratification factors included institution, prior taxane-based therapy, involved axillary lymph nodes, and centrally determined phenotype (basal v nonbasal, according to cytokeratins 5/6 and/or epidermal growth factor receptor positivity by immunohistochemistry). The primary objective was to compare disease-free survival (DFS) between both arms.
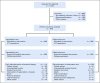
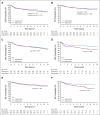
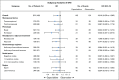
Results: Eight hundred seventy-six patients were randomly assigned to capecitabine (n = 448) or observation (n = 428). Median age was 49 years, 55.9% were lymph node negative, 73.9% had a basal phenotype, and 67.5% received previous anthracyclines plus taxanes. Median length of follow-up was 7.3 years. DFS was not significantly prolonged with capecitabine versus observation [hazard ratio (HR), 0.82; 95% CI, 0.63 to 1.06; P = .136]. In a preplanned subgroup analysis, nonbasal patients seemed to derive benefit from the addition of capecitabine with a DFS HR of 0.53 versus 0.94 in those with basal phenotype (interaction test P = .0694) and an HR for overall survival of 0.42 versus 1.23 in basal phenotype (interaction test P = .0052). Tolerance of capecitabine was as expected, with 75.2% of patients completing the planned 8 cycles.
Conclusion: This study failed to show a statistically significant increase in DFS by adding extended capecitabine to standard chemotherapy in patients with early TNBC. In a preplanned subset analysis, patients with nonbasal phenotype seemed to obtain benefit with capecitabine, although this will require additional validation.
Figures
Comment in
-
Role of Capecitabine in Early Breast Cancer.J Clin Oncol. 2020 Jan 20;38(3):179-182. doi: 10.1200/JCO.19.02946. Epub 2019 Dec 5. J Clin Oncol. 2020. PMID: 31804861 Free PMC article. No abstract available.
-
Reply to Y. Usui et al.J Clin Oncol. 2020 Jun 20;38(18):2113-2114. doi: 10.1200/JCO.20.00390. Epub 2020 Apr 30. J Clin Oncol. 2020. PMID: 32352858 No abstract available.
-
Suggestions Regarding the GEICAM/2003-11_CIBOMA/2004-01 Trial: Future Treatment Options for Early Triple-Negative Breast Cancer.J Clin Oncol. 2020 Jun 20;38(18):2111-2112. doi: 10.1200/JCO.19.03406. Epub 2020 Apr 30. J Clin Oncol. 2020. PMID: 32352859 No abstract available.
References
-
- Li X, Yang J, Peng L, et al. Triple-negative breast cancer has worse overall survival and cause-specific survival than non-triple-negative breast cancer. Breast Cancer Res Treat. 2017;161:279–287. - PubMed
-
- Walko CM, Lindley C. Capecitabine: A review. Clin Ther. 2005;27:23–44. - PubMed
-
- Cheang MC, Voduc D, Bajdik C, et al. Basal-like breast cancer defined by five biomarkers has superior prognostic value than triple-negative phenotype. Clin Cancer Res. 2008;14:1368–1376. - PubMed
-
- GEICAM Spanish Breast Cancer Group . Proyecto El Álamo III. Encuesta de Evolución de Pacientes Con Cáncer de Mama en Hospitales del Grupo GEICAM (1998-2001) Madrid, Spain: GEICAM Spanish Breast Cancer Group; 2014.
-
- Martín M, Ruiz Simón A, Ruiz Borrego M, et al. Epirubicin plus cyclophosphamide followed by docetaxel versus epirubicin plus docetaxel followed by capecitabine as adjuvant therapy for node-positive early breast cancer: Results from the GEICAM/2003-10 study. J Clin Oncol. 2015;33:3788–3795. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

