DGCR8 microprocessor defect characterizes familial multinodular goiter with schwannomatosis
- PMID: 31805011
- PMCID: PMC7269565
- DOI: 10.1172/JCI130206
DGCR8 microprocessor defect characterizes familial multinodular goiter with schwannomatosis
Abstract
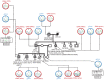
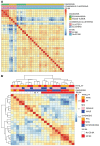
BACKGROUNDDICER1 is the only miRNA biogenesis component associated with an inherited tumor syndrome, featuring multinodular goiter (MNG) and rare pediatric-onset lesions. Other susceptibility genes for familial forms of MNG likely exist.METHODSWhole-exome sequencing of a kindred with early-onset MNG and schwannomatosis was followed by investigation of germline pathogenic variants that fully segregated with the disease. Genome-wide analyses were performed on 13 tissue samples from familial and nonfamilial DGCR8-E518K-positive tumors, including MNG, schwannomas, papillary thyroid cancers (PTCs), and Wilms tumors. miRNA profiles of 4 tissue types were compared, and sequencing of miRNA, pre-miRNA, and mRNA was performed in a subset of 9 schwannomas, 4 of which harbor DGCR8-E518K.RESULTSWe identified c.1552G>A;p.E518K in DGCR8, a microprocessor component located in 22q, in the kindred. The variant identified is a somatic hotspot in Wilms tumors and has been identified in 2 PTCs. Copy number loss of chromosome 22q, leading to loss of heterozygosity at the DGCR8 locus, was found in all 13 samples harboring c.1552G>A;p.E518K. miRNA profiling of PTCs, MNG, schwannomas, and Wilms tumors revealed a common profile among E518K hemizygous tumors. In vitro cleavage demonstrated improper processing of pre-miRNA by DGCR8-E518K. MicroRNA and RNA profiling show that this variant disrupts precursor microRNA production, impacting populations of canonical microRNAs and mirtrons.CONCLUSIONWe identified DGCR8 as the cause of an unreported autosomal dominant mendelian tumor susceptibility syndrome: familial multinodular goiter with schwannomatosis.FUNDINGCanadian Institutes of Health Research, Compute Canada, Alex's Lemonade Stand Foundation, the Mia Neri Foundation for Childhood Cancer, Cassa di Sovvenzioni e Risparmio fra il Personale della Banca d'Italia, and the KinderKrebsInitiative Buchholz/Holm-Seppensen.
Keywords: Genetic diseases; Genetics; Oncology; RNA processing; Thyroid disease.
Conflict of interest statement
Figures


References
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

