Safety of dabigatran etexilate for the secondary prevention of venous thromboembolism in children
- PMID: 31805182
- PMCID: PMC7019192
- DOI: 10.1182/blood.2019000998
Safety of dabigatran etexilate for the secondary prevention of venous thromboembolism in children
Erratum in
-
Brandão LR, Albisetti M, Halton J, et al; DIVERSITY Study Investigators. Safety of dabigatran etexilate for the secondary prevention of venous thromboembolism in children. Blood. 2020;135(7):491-504.Blood. 2020 May 7;135(19):1720. doi: 10.1182/blood.2020005941. Blood. 2020. PMID: 32379879 Free PMC article. No abstract available.
Abstract
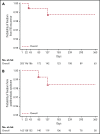
This open-label, single-arm, prospective cohort trial is the first phase 3 safety study to describe outcomes in children treated with dabigatran etexilate for secondary venous thromboembolism (VTE) prevention. Eligible children aged 12 to <18 years (age stratum 1), 2 to <12 years (stratum 2), and >3 months to <2 years (stratum 3) had an objectively confirmed diagnosis of VTE treated with standard of care (SOC) for ≥3 months, or had completed dabigatran or SOC treatment in the DIVERSITY trial (NCT01895777) and had an unresolved clinical thrombosis risk factor requiring further anticoagulation. Children received dabigatran for up to 12 months, or less if the identified VTE clinical risk factor resolved. Primary end points included VTE recurrence, bleeding events, and mortality at 6 and 12 months. Overall, 203 children received dabigatran, with median exposure being 36.3 weeks (range, 0-57 weeks); 171 of 203 (84.2%) and 32 of 203 (15.8%) took capsules and pellets, respectively. Overall, 2 of 203 children (1.0%) experienced on-treatment VTE recurrence, and 3 of 203 (1.5%) experienced major bleeding events, with 2 (1.0%) reporting clinically relevant nonmajor bleeding events, and 37 (18.2%) minor bleeding events. There were no on-treatment deaths. On-treatment postthrombotic syndrome was reported for 2 of 162 children (1.2%) who had deep vein thrombosis or central-line thrombosis as their most recent VTE. Pharmacokinetic/pharmacodynamic relationships of dabigatran were similar to those in adult VTE patients. In summary, dabigatran showed a favorable safety profile for secondary VTE prevention in children aged from >3 months to <18 years with persistent VTE risk factor(s). This trial was registered at www.clinicaltrials.gov as #NCT02197416.
© 2020 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: L.R.B. is a member of a Pediatric Expert Working Group for Boehringer Ingelheim, and has received Advisory Board fees from Boehringer Ingelheim. M.A. is a member of a Pediatric Expert Working Group for Boehringer Ingelheim, and has received Advisory Board fees from Daiichi Sankyo. J.H. is a member of a Pediatric Expert Working Group for Boehringer Ingelheim and has received honoraria from Boehringer Ingelheim for congress presentation. L.B. is a member of a Pediatric Expert Working Group for Boehringer Ingelheim, and reports fees to her institution from Janssen Pharmaceuticals. E.C. is a member of a Pediatric Expert Working Group for Boehringer Ingelheim, and reports personal fees from Roche, Sobi, Bristol-Myers Squibb, CSL Behring, and Shire/Takeda. L.G.M. is a member of a Pediatric Expert Working Group for Boehringer Ingelheim, reports consultant fees from Pfizer as a steering committee member, and has received a research grant from Bristol-Myers Squibb. P.S. reports personal fees from Takeda and CSL Behring. I.T., M.S., F.H., Z.S., J.K., S.G., and M.B. are all employees of Boehringer Ingelheim. M.L. is a member of a Pediatric Expert Working Group for Boehringer Ingelheim. The remaining authors declare no competing financial interests.
Figures



Comment in
-
The phase 3 pediatric anticoagulant era.Blood. 2020 Feb 13;135(7):459-460. doi: 10.1182/blood.2019004340. Blood. 2020. PMID: 32197268 Free PMC article.
References
-
- Andrew M, David M, Adams M, et al. . Venous thromboembolic complications (VTE) in children: first analyses of the Canadian Registry of VTE. Blood. 1994;83(5):1251-1257. - PubMed
-
- Nowak-Göttl U, Junker R, Kreuz W, et al. ; Childhood Thrombophilia Study Group . Risk of recurrent venous thrombosis in children with combined prothrombotic risk factors. Blood. 2001;97(4):858-862. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

