Sustained low-dose dexamethasone delivery via a PLGA microsphere-embedded agarose implant for enhanced osteochondral repair
- PMID: 31805408
- PMCID: PMC6956850
- DOI: 10.1016/j.actbio.2019.11.052
Sustained low-dose dexamethasone delivery via a PLGA microsphere-embedded agarose implant for enhanced osteochondral repair
Abstract
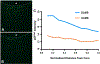
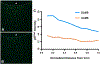
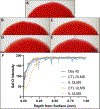
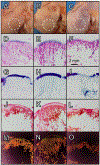
Articular cartilage defects are a common source of joint pain and dysfunction. We hypothesized that sustained low-dose dexamethasone (DEX) delivery via an acellular osteochondral implant would have a dual pro-anabolic and anti-catabolic effect, both supporting the functional integrity of adjacent graft and host tissue while also attenuating inflammation caused by iatrogenic injury. An acellular agarose hydrogel carrier with embedded DEX-loaded poly(lactic-co-glycolic) acid (PLGA) microspheres (DLMS) was developed to provide sustained release for at least 99 days. The DLMS implant was first evaluated in an in vitro pro-inflammatory model of cartilage degradation. The implant was chondroprotective, as indicated by maintenance of Young's modulus (EY) (p = 0.92) and GAG content (p = 1.0) in the presence of interleukin-1β insult. In a subsequent preliminary in vivo experiment, an osteochondral autograft transfer was performed using a pre-clinical canine model. DLMS implants were press-fit into the autograft donor site and compared to intra-articular DEX injection (INJ) or no DEX (CTL). Functional scores for DLMS animals returned to baseline (p = 0.39), whereas CTL and INJ remained significantly worse at 6 months (p < 0.05). DLMS knees were significantly more likely to have improved OARSI scores for proteoglycan, chondrocyte, and collagen pathology (p < 0.05). However, no significant improvements in synovial fluid cytokine content were observed. In conclusion, utilizing a targeted DLMS implant, we observed in vitro chondroprotection in the presence of IL-1-induced degradation and improved in vivo functional outcomes. These improved outcomes were correlated with superior histological scores but not necessarily a dampened inflammatory response, suggesting a primarily pro-anabolic effect. STATEMENT OF SIGNIFICANCE: Articular cartilage defects are a common source of joint pain and dysfunction. Effective treatment of these injuries may prevent the progression of osteoarthritis and reduce the need for total joint replacement. Dexamethasone, a potent glucocorticoid with concomitant anti-catabolic and pro-anabolic effects on cartilage, may serve as an adjuvant for a variety of repair strategies. Utilizing a dexamethasone-loaded osteochondral implant with controlled release characteristics, we demonstrated in vitro chondroprotection in the presence of IL-1-induced degradation and improved in vivo functional outcomes following osteochondral repair. These improved outcomes were correlated with superior histological cartilage scores and minimal-to-no comorbidity, which is a risk with high dose dexamethasone injections. Using this model of cartilage restoration, we have for the first time shown the application of targeted, low-dose dexamethasone for improved healing in a preclinical model of focal defect repair.
Keywords: Dexamethasone; Microspheres; Osteochondral repair; Preclinical models; Targeted drug delivery.
Copyright © 2019. Published by Elsevier Ltd.
Conflict of interest statement
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

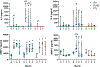
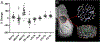

References
-
- Allen DB. GROWTH SUPPRESSION BY GLUCOCORTICOID THERAPY. Endocrinol. Metab. Clin. North Am 1996;25:699–717. - PubMed
-
- Amin AK, Simpson AHRW, Hall AC. Iatrogenic articular cartilage injury: the elephant in the operating theatre. Bone Jt. J 2017;99-B:1555–1556. - PubMed
-
- Anderson JM, Shive MS. Biodegradation and biocompatibility of PLA and PLGA microspheres. Adv. Drug Deliv. Rev 2012;64:72–82. - PubMed
-
- Anon. Dexamethasone Sodium Phosphate Injection, USP: 2014.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

