Bioengineered Skin Substitutes: the Role of Extracellular Matrix and Vascularization in the Healing of Deep Wounds
- PMID: 31805652
- PMCID: PMC6947552
- DOI: 10.3390/jcm8122083
Bioengineered Skin Substitutes: the Role of Extracellular Matrix and Vascularization in the Healing of Deep Wounds
Abstract
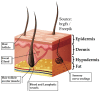
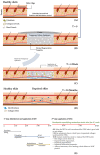
The formation of severe scars still represents the result of the closure process of extended and deep skin wounds. To address this issue, different bioengineered skin substitutes have been developed but a general consensus regarding their effectiveness has not been achieved yet. It will be shown that bioengineered skin substitutes, although representing a valid alternative to autografting, induce skin cells in repairing the wound rather than guiding a regeneration process. Repaired skin differs from regenerated skin, showing high contracture, loss of sensitivity, impaired pigmentation and absence of cutaneous adnexa (i.e., hair follicles and sweat glands). This leads to significant mobility and aesthetic concerns, making the development of more effective bioengineered skin models a current need. The objective of this review is to determine the limitations of either commercially available or investigational bioengineered skin substitutes and how advanced skin tissue engineering strategies can be improved in order to completely restore skin functions after severe wounds.
Keywords: bioreactors; bottom-up tissue engineering; dermal substitutes; extracellular matrix; scar tissue.; skin substitutes; tissue engineering; vascularization; wound healing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Kanitakis J. Anatomy, histology and immunohistochemistry of normal human skin. Eur. J. Dermatol. 2002;12:390–401. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

