Generation and Immunogenicity of a Recombinant Pseudorabies Virus Co-Expressing Classical Swine Fever Virus E2 Protein and Porcine Circovirus Type 2 Capsid Protein Based on Fosmid Library Platform
- PMID: 31805703
- PMCID: PMC6963705
- DOI: 10.3390/pathogens8040279
Generation and Immunogenicity of a Recombinant Pseudorabies Virus Co-Expressing Classical Swine Fever Virus E2 Protein and Porcine Circovirus Type 2 Capsid Protein Based on Fosmid Library Platform
Abstract
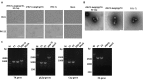
Pseudorabies (PR), classical swine fever (CSF), and porcine circovirus type 2 (PCV2)-associated disease (PCVAD) are economically important infectious diseases of pigs. Co-infections of these diseases often occur in the field, posing significant threat to the swine industry worldwide. gE/gI/TK-gene-deleted vaccines are safe and capable of providing full protection against PR. Classical swine fever virus (CSFV) E2 glycoprotein is mainly used in the development of CSF vaccines. PCV2 capsid (Cap) protein is the major antigen targeted for developing PCV2 subunit vaccines. Multivalent vaccines, and especially virus-vectored vaccines expressing foreign proteins, are attractive strategies to fight co-infections for various swine diseases. The gene-deleted pseudorabies virus (PRV) can be used to develop promising and economical multivalent live virus-vectored vaccines. Herein, we constructed a gE/gI/TK-gene-deleted PRV co-expressing E2 of CSFV and Cap of PCV2 by fosmid library platform established for PRV, and the expression of E2 and Cap proteins was confirmed using immunofluorescence assay and western blotting. The recombinant virus propagated in porcine kidney 15 (PK-15) cells for 20 passages was genetically stable. The evaluation results in rabbits and pigs demonstrate that rPRVTJ-delgE/gI/TK-E2-Cap elicited detectable anti-PRV antibodies, but not anti-PCV2 or anti-CSFV antibodies. These findings provide insights that rPRVTJ-delgE/gI/TK-E2-Cap needs to be optimally engineered as a promising trivalent vaccine candidate against PRV, PCV2 and CSFV co-infections in future.
Keywords: E2 and Cap; classical swine fever virus; fosmid; porcine circovirus type 2; pseudorabies virus.
Conflict of interest statement
Authors declare no conflicts of interest.
Figures
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

