Aging and Central Auditory Disinhibition: Is It a Reflection of Homeostatic Downregulation or Metabolic Vulnerability?
- PMID: 31805729
- PMCID: PMC6955996
- DOI: 10.3390/brainsci9120351
Aging and Central Auditory Disinhibition: Is It a Reflection of Homeostatic Downregulation or Metabolic Vulnerability?
Abstract
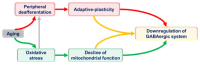
Aging-related changes have been identified at virtually every level of the central auditory system. One of the most common findings across these nuclei is a loss of synaptic inhibition with aging, which has been proposed to be at the heart of several aging-related changes in auditory cognition, including diminished speech perception in complex environments and the presence of tinnitus. Some authors have speculated that downregulation of synaptic inhibition is a consequence of peripheral deafferentation and therefore is a homeostatic mechanism to restore excitatory/inhibitory balance. As such, disinhibition would represent a form of maladaptive plasticity. However, clinical data suggest that deafferentation-related disinhibition tends to occur primarily in the aged brain. Therefore, aging-related disinhibition may, in part, be related to the high metabolic demands of inhibitory neurons relative to their excitatory counterparts. These findings suggest that both deafferentation-related maladaptive plastic changes and aging-related metabolic factors combine to produce changes in central auditory function. Here, we explore the arguments that downregulation of inhibition may be due to homeostatic responses to diminished afferent input vs. metabolic vulnerability of inhibitory neurons in the aged brain. Understanding the relative importance of these mechanisms will be critical for the development of treatments for the underlying causes of aging-related central disinhibition.
Keywords: GABA; aging; auditory; glycine; metabolism; mitochondria; presbycusis; tinnitus.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Cruickshanks K.J., Zhan W., Zhong W. Epidemiology of Age-Related Hearing Impairment. In: Gordon-Salant S., Frisina R.D., Popper A.N.F., Richard R., editors. The Aging Auditory System. Volume 34. Springer; New York, NY, USA: 2010. pp. 259–274.
-
- Ortman J.M., Velkoff V.A., Hogan H. An Aging Nation: The Older Population in the United States. US Census Bureau; Washington, DC, USA: 2014. pp. 25–1140.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

