HER2 gene (ERBB2) amplification is a rare event in non-liver-fluke associated cholangiocarcinogenesis
- PMID: 31805897
- PMCID: PMC6896712
- DOI: 10.1186/s12885-019-6320-y
HER2 gene (ERBB2) amplification is a rare event in non-liver-fluke associated cholangiocarcinogenesis
Abstract
Background: Cholangiocarcinoma is a rapidly fatal cancer entity with a median survival of less than one year. In contrast to many other malignancies, no substantial therapeutic breakthrough has been made in the past few decades, thereby limiting the treatment to cytotoxic chemotherapy with little beneficial effect for most patients. Targeted therapy tailored to the individual has shown substantial success in the recent past as a promising avenue for cancer therapy.
Methods: In this study, we determined the frequency of amplification of the HER2 gene in a comprehensive and well-characterized European cholangiocarcinoma cohort encompassing 436 patients including intrahepatic (n = 155), proximal (n = 155) and distal (n = 126) cholangiocarcinoma by strict application of a combined immunohistochemical and in situ hybridization algorithm following the current guidelines for HER2 assessment in gastric cancer.
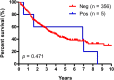
Results: We identified a proportion of 1.4% (n = 6) patients that demonstrated HER2 gene amplification, with the highest rate among the distal cholangiocarcinoma patients (2.4%). None of the patients with equivocal (2+) immunohistochemical staining results exhibited gene amplification molecularly. In four of the five patients with HER2 positivity, gene amplification was already present in concomitantly tested high-grade biliary intraepithelial neoplasia (80%). HER2 gene amplification was not significantly associated with other clinical parameters, including survival.
Conclusions: This study identifies HER2 gene amplification as a rare event in cholangiocarcinoma of the Western population, occurring already in high-grade BilIN in a subset of patients. Furthermore, we provide a robust testing algorithm that may be used prior to therapy administration in future clinical trials evaluating the role of HER2 as a predictive marker in cholangiocarcinoma.
Keywords: Biliary tract cancer; Cholangiocarcinoma; HER2; Predictive biomarkers; Targeted therapy.
Conflict of interest statement
PS received funding for grants, boards, and presentations from Novartis. The other authors declare no competing interests.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

