Changing plasma cytokine, chemokine and growth factor profiles upon differing malaria transmission intensities
- PMID: 31806027
- PMCID: PMC6896751
- DOI: 10.1186/s12936-019-3038-x
Changing plasma cytokine, chemokine and growth factor profiles upon differing malaria transmission intensities
Abstract
Background: Malaria epidemiological and immunological data suggest that parasite tolerance wanes in the absence of continuous exposure to the parasite, potentially enhancing pathogenesis. The expansion of control interventions and elimination campaigns raises the necessity to better understand the host factors leading to susceptibility or tolerance that are affected by rapid changes in malaria transmission intensity (MTI). Mediators of cellular immune responses are responsible for the symptoms and pathological alterations during disease and are expected to change rapidly upon malaria exposure or cessation.
Methods: The plasma concentrations of 30 cytokine, chemokine and growth factors in individuals of all ages from a malaria endemic area of southern Mozambique were compared between 2 years of different MTI: 2010 (lower, n = 234) and 2013 (higher, n = 143). The effect of the year on the correlations between cytokines, chemokines and growth factors and IgGs to Plasmodium falciparum (markers of exposure) was explored. The effects of age, sex, neighbourhood and parasitaemia on analyte levels and their interactions with year were also assessed.
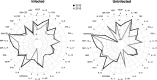
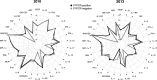
Results: An inverse correlation of several cellular immune mediators with malarial antibodies in 2013, and a lack of correlation or even a positive correlation in 2010 were observed. Most cytokines, chemokines and growth factors, regardless of their immune function, had higher concentrations in 2010 compared with 2013 in P. falciparum-infected and uninfected subjects. Age and neighbourhood showed an effect on analyte concentrations.
Conclusions: The results show a different regulation of the cellular immune response in 2010 vs 2013 which could be related to a loss of immune-tolerance after a decline in MTI in 2010 and previous years, and a rapid re-establishment of tolerance as a consequence of more continuous exposure as MTI began increasing in 2012. Cellular immune mediators warrant further investigation as possible surrogates of MTI-associated host susceptibility or tolerance.
Keywords: Age; Antibodies; Chemokines; Cytokines; Growth factors; Immunity; Malaria transmission intensity; Plasmodium falciparum; Tolerance.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
-
- WHO. World malaria report 2018. Geneva: World Health Organization; 2018. https://www.who.int/malaria/publications/world-malaria-report-2018/en/. Accessed 1 Apr 2019

