Aurora B functions at the apical surface after specialized cytokinesis during morphogenesis in C. elegans
- PMID: 31806662
- PMCID: PMC6983721
- DOI: 10.1242/dev.181099
Aurora B functions at the apical surface after specialized cytokinesis during morphogenesis in C. elegans
Abstract
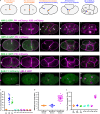
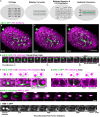
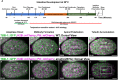
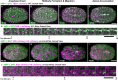
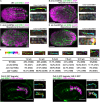
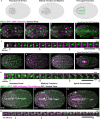
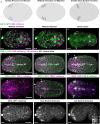
Although cytokinesis has been intensely studied, the way it is executed during development is not well understood, despite a long-standing appreciation that various aspects of cytokinesis vary across cell and tissue types. To address this, we investigated cytokinesis during the invariant Caenorhabditis elegans embryonic divisions and found several parameters that are altered at different stages in a reproducible manner. During early divisions, furrow ingression asymmetry and midbody inheritance is consistent, suggesting specific regulation of these events. During morphogenesis, we found several unexpected alterations to cytokinesis, including apical midbody migration in polarizing epithelial cells of the gut, pharynx and sensory neurons. Aurora B kinase, which is essential for several aspects of cytokinesis, remains apically localized in each of these tissues after internalization of midbody ring components. Aurora B inactivation disrupts cytokinesis and causes defects in apical structures, even if inactivated post-mitotically. Therefore, we demonstrate that cytokinesis is implemented in a specialized way during epithelial polarization and that Aurora B has a role in the formation of the apical surface.
Keywords: Apical surface; Aurora B kinase; Cytokinesis; Midbody; Morphogenesis.
© 2020. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures

References
-
- Amini R., Goupil E., Labella S., Zetka M., Maddox A. S., Labbe J. C. and Chartier N. T. (2015). C. elegans Anillin proteins regulate intercellular bridge stability and germline syncytial organization (vol 206, pg 129, 2014). J. Cell Biol. 209, 467-467. 10.1083/jcb.20131011704212015c - DOI - PMC - PubMed
-
- Bernabé-Rubio M., Andrés G., Casares-Arias J., Fernández-Barrera J., Rangel L., Reglero-Real N., Gershlick D. C., Fernández J. J., Millán J., Correas I. et al. (2016). Novel role for the midbody in primary ciliogenesis by polarized epithelial cells. J. Cell Biol. 214, 259-273. 10.1083/jcb.201601020 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

