Understanding intrinsic hematopoietic stem cell aging
- PMID: 31806687
- PMCID: PMC6939535
- DOI: 10.3324/haematol.2018.211342
Understanding intrinsic hematopoietic stem cell aging
Abstract
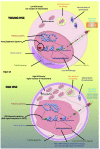
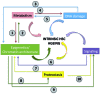
Hematopoietic stem cells (HSC) sustain blood production over the entire life-span of an organism. It is of extreme importance that these cells maintain self-renewal and differentiation potential over time in order to preserve homeostasis of the hematopoietic system. Many of the intrinsic aspects of HSC are affected by the aging process resulting in a deterioration in their potential, independently of their microenvironment. Here we review recent findings characterizing most of the intrinsic aspects of aged HSC, ranging from phenotypic to molecular alterations. Historically, DNA damage was thought to be the main cause of HSC aging. However, over recent years, many new findings have defined an increasing number of biological processes that intrinsically change with age in HSC. Epigenetics and chromatin architecture, together with autophagy, proteostasis and metabolic changes, and how they are interconnected, are acquiring growing importance for understanding the intrinsic aging of stem cells. Given the increase in populations of older subjects worldwide, and considering that aging is the primary risk factor for most diseases, understanding HSC aging becomes particularly relevant also in the context of hematologic disorders, such as myelodysplastic syndromes and acute myeloid leukemia. Research on intrinsic mechanisms responsible for HSC aging is providing, and will continue to provide, new potential molecular targets to possibly ameliorate or delay aging of the hematopoietic system and consequently improve the outcome of hematologic disorders in the elderly. The niche-dependent contributions to hematopoietic aging are discussed in another review in this same issue of the Journal.
Copyright© 2020 Ferrata Storti Foundation.
Figures


References
-
- Meyers J, Yu Y, Kaye JA, Davis KL. Medicare fee-for-service enrollees with primary acute myeloid leukemia: an analysis of treatment patterns, survival, and healthcare resource utilization and costs. Appl Health Econ Health Policy. 2013;11(3):275–286. - PubMed
-
- Chandel NS, Jasper H, Ho TT, Passegue E. Metabolic regulation of stem cell function in tissue homeostasis and organismal ageing. Nat Cell Biol. 2016;18(8):823–832. - PubMed

