Lipid-gated monovalent ion fluxes regulate endocytic traffic and support immune surveillance
- PMID: 31806695
- PMCID: PMC8118712
- DOI: 10.1126/science.aaw9544
Lipid-gated monovalent ion fluxes regulate endocytic traffic and support immune surveillance
Abstract
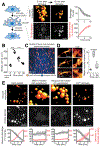
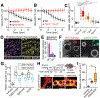
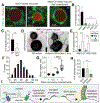
Despite ongoing (macro)pinocytosis of extracellular fluid, the volume of the endocytic pathway remains unchanged. To investigate the underlying mechanism, we used high-resolution video imaging to analyze the fate of macropinosomes formed by macrophages in vitro and in situ. Na+, the primary cationic osmolyte internalized, exited endocytic vacuoles via two-pore channels, accompanied by parallel efflux of Cl- and osmotically coupled water. The resulting shrinkage caused crenation of the membrane, which fostered recruitment of curvature-sensing proteins. These proteins stabilized tubules and promoted their elongation, driving vacuolar remodeling, receptor recycling, and resolution of the organelles. Failure to resolve internalized fluid impairs the tissue surveillance activity of resident macrophages. Thus, osmotically driven increases in the surface-to-volume ratio of endomembranes promote traffic between compartments and help to ensure tissue homeostasis.
Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Figures

Comment in
-
Water loss regulates cell and vesicle volume.Science. 2020 Jan 17;367(6475):246-247. doi: 10.1126/science.aba3623. Science. 2020. PMID: 31949066 No abstract available.
References
-
- Swanson JA, Watts C, Macropinocytosis. Trends Cell Biol 5, 424–428 (1995). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

