Increased stiffness and flow resistance of the inner wall of Schlemm's canal in glaucomatous human eyes
- PMID: 31806762
- PMCID: PMC6936716
- DOI: 10.1073/pnas.1911837116
Increased stiffness and flow resistance of the inner wall of Schlemm's canal in glaucomatous human eyes
Abstract
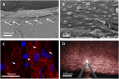
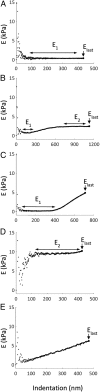
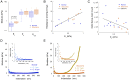
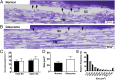
The cause of the elevated outflow resistance and consequent ocular hypertension characteristic of glaucoma is unknown. To investigate possible causes for this flow resistance, we used atomic force microscopy (AFM) with 10-µm spherical tips to probe the stiffness of the inner wall of Schlemm's canal as a function of distance from the tissue surface in normal and glaucomatous postmortem human eyes, and 1-µm spherical AFM tips to probe the region immediately below the tissue surface. To localize flow resistance, perfusion and imaging methods were used to characterize the pressure drop in the immediate vicinity of the inner wall using giant vacuoles that form in Schlemm's canal cells as micropressure sensors. Tissue stiffness increased with increasing AFM indentation depth. Tissues from glaucomatous eyes were stiffer compared with normal eyes, with greatly increased stiffness residing within ∼1 µm of the inner-wall surface. Giant vacuole size and density were similar in normal and glaucomatous eyes despite lower flow rate through the latter due to their higher flow resistance. This implied that the elevated flow resistance found in the glaucomatous eyes was localized to the same region as the increased tissue stiffness. Our findings implicate pathological changes to biophysical characteristics of Schlemm's canal endothelia and/or their immediate underlying extracellular matrix as cause for ocular hypertension in glaucoma.
Keywords: biophysics; extracellular matrix; primary open-angle glaucoma.
Conflict of interest statement
The authors declare no competing interest.
Figures

References
-
- Leber T., Studien über den Flüssigkeitswechsel im Auge. Albrecht Von Graefes Arch. Ophthalmol. 19, 87–106 (1873).
-
- Grant W. M., Clinical measurements of aqueous outflow. AMA Arch. Opthalmol. 46, 113–131 (1951). - PubMed
-
- Johnson M., Erickson K., “Mechanisms and routes of aqueous humor drainage” in Principles and Practice of Ophthalmology, Albert D. M., Jakobiec F. A., Eds. (WB Saunders, Philadelphia, 2000), vol. 4 Glaucoma, chap. 193B, pp. 2577–2595.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

