Undulating changes in human plasma proteome profiles across the lifespan
- PMID: 31806903
- PMCID: PMC7062043
- DOI: 10.1038/s41591-019-0673-2
Undulating changes in human plasma proteome profiles across the lifespan
Abstract
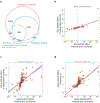
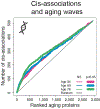
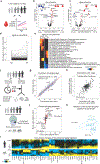
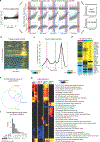
Aging is a predominant risk factor for several chronic diseases that limit healthspan1. Mechanisms of aging are thus increasingly recognized as potential therapeutic targets. Blood from young mice reverses aspects of aging and disease across multiple tissues2-10, which supports a hypothesis that age-related molecular changes in blood could provide new insights into age-related disease biology. We measured 2,925 plasma proteins from 4,263 young adults to nonagenarians (18-95 years old) and developed a new bioinformatics approach that uncovered marked non-linear alterations in the human plasma proteome with age. Waves of changes in the proteome in the fourth, seventh and eighth decades of life reflected distinct biological pathways and revealed differential associations with the genome and proteome of age-related diseases and phenotypic traits. This new approach to the study of aging led to the identification of unexpected signatures and pathways that might offer potential targets for age-related diseases.
Conflict of interest statement
Competing Interests
The authors declare no competing financial interests.
Figures








References
Methods-only References
-
- Franceschi C, et al. Genetics of healthy aging in Europe: the EU-integrated project GEHA (GEnetics of Healthy Aging). Ann N Y Acad Sci 1100, 21–45 (2007). - PubMed
-
- Fox J & Weisberg S An R Companion to Applied Regression, (SAGE Publications, 2011).
-
- Benjamini Y & Hochberg Y Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society. Series B (Methodological) 57, 289–300 (1995).
Publication types
MeSH terms
Substances
Grants and funding
- RG/18/13/33946/BHF_/British Heart Foundation/United Kingdom
- RG/13/13/30194/BHF_/British Heart Foundation/United Kingdom
- K99 NS112458/NS/NINDS NIH HHS/United States
- R01 AG044829/AG/NIA NIH HHS/United States
- DP1 AG053015/AG/NIA NIH HHS/United States
- P30 AG038072/AG/NIA NIH HHS/United States
- T32 AG047126/AG/NIA NIH HHS/United States
- R00 NS112458/NS/NINDS NIH HHS/United States
- F32 AG055255/AG/NIA NIH HHS/United States
- R01 AG045034/AG/NIA NIH HHS/United States
- R01 AG057909/AG/NIA NIH HHS/United States
- MR/L003120/1/MRC_/Medical Research Council/United Kingdom
- R01 AG061155/AG/NIA NIH HHS/United States
- P50 AG047366/AG/NIA NIH HHS/United States
- K23 AG051148/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

