GLUT-1 siRNA Enhances Radiosensitization Of Laryngeal Cancer Stem Cells Via Enhanced DNA Damage, Cell Cycle Redistribution, And Promotion Of Apoptosis In Vitro And In Vivo
- PMID: 31806998
- PMCID: PMC6842317
- DOI: 10.2147/OTT.S221423
GLUT-1 siRNA Enhances Radiosensitization Of Laryngeal Cancer Stem Cells Via Enhanced DNA Damage, Cell Cycle Redistribution, And Promotion Of Apoptosis In Vitro And In Vivo
Abstract
Background: Radiotherapy does not show good efficacy against laryngeal cancer due to radioresistance. Cancer stem cells (CSCs) are considered among the causes of radioresistance. Inhibition of glucose transporter-1 (GLUT-1) using GLUT-1 small interfering RNA (siRNA) may enhance the radiosensitivity of laryngeal cancer cells, but the underlying cellular mechanisms remain unclear.
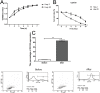
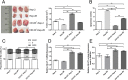
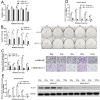
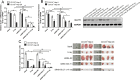
Methods: The CD133+-Hep-2R cell line was established with repeated irradiation and magnetic-activated cell sorting. The effects of irradiation on CD133+-Hep-2R cells were examined by CCK-8 assay, Transwell assay, quantitative real-time polymerase chain reaction (RT-PCR), and Western blotting. The effects of GLUT-1 siRNA on the radiosensitivity of CD133+-Hep-2/2R cells were examined by RT-PCR, Western blotting, CCK-8 assay, colony formation assay, and Transwell assay in vitro and in a xenograft tumor model in nude mice. The cellular mechanism of enhanced radiosensitivity associated with GLUT-1 siRNA was investigated. The cell cycle and apoptosis rate were analyzed by flow cytometry, and the repair capability was examined by determining the levels of RAD51 and DNA-PKcs.
Results: CD133+-Hep-2/2R cells showed stronger proliferation, lower apoptosis rate, lower percentage of G0/G1 phase cells, higher percentages of S and G2/M phase cells, and higher expression levels of GLUT-1 than Hep-2/2R cells. Transfection with GLUT-1 siRNA inhibited the proliferation and invasive capability of CD133+-Hep-2R cells by inhibiting GLUT-1 expression, which also caused a redistribution of the cell cycle (higher proportion of cells in the G0/G1 phase and lower proportion in the S and G2/M phases), increased the apoptosis rate, and reduced DNA repair capability by suppressing RAD51 and DNA-PKcs expression.
Conclusion: The results of this study suggest that GLUT-1 siRNA can enhance the radiosensitivity of CD133+-Hep-2R cells by inducing a redistribution of cell cycle phases, inhibiting DNA repair capability, and increasing apoptosis. Inhibition of GLUT-1 may have therapeutic potential for interventions to increase the radiosensitivity of laryngeal CSCs.
Keywords: DNA double-strand break; glucose transporter-1; laryngeal cancer stem cells; radioresistance; small interfering RNA.
© 2019 Zhong et al.
Conflict of interest statement
The authors declare that they have no competing interests in this work.
Figures

References
-
- Steuer CE, El-Deiry M, Parks JR, et al. An update on larynx cancer. Cancer J Clin. 2017;67(1):31–50. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

