Emission solvatochromic, solid-state and aggregation-induced emissive α-pyrones and emission-tuneable 1 H-pyridines by Michael addition-cyclocondensation sequences
- PMID: 31807204
- PMCID: PMC6880829
- DOI: 10.3762/bjoc.15.262
Emission solvatochromic, solid-state and aggregation-induced emissive α-pyrones and emission-tuneable 1 H-pyridines by Michael addition-cyclocondensation sequences
Abstract
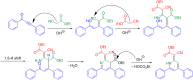
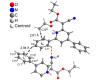
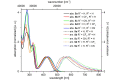
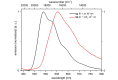
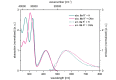
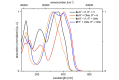
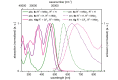
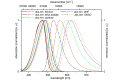
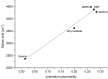
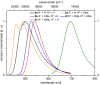
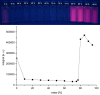
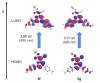
Starting from substituted alkynones, α-pyrones and/or 1H-pyridines were generated in a Michael addition-cyclocondensation with ethyl cyanoacetate. The peculiar product formation depends on the reaction conditions as well as on the electronic substitution pattern of the alkynone. While electron-donating groups furnish α-pyrones as main products, electron-withdrawing groups predominantly give the corresponding 1H-pyridines. Both heterocycle classes fluoresce in solution and in the solid state. In particular, dimethylamino-substituted α-pyrones, as donor-acceptor systems, display remarkable photophysical properties, such as strongly red-shifted absorption and emission maxima with daylight fluorescence and fluorescence quantum yields up to 99% in solution and around 11% in the solid state, as well as pronounced emission solvatochromism. Also a donor-substituted α-pyrone shows pronounced aggregation-induced emission enhancement.
Keywords: 1H-pyridines; DFT calculations; cyclocondensation; fluorescence; heterocycles; α-pyrones.
Copyright © 2019, Breuer et al.; licensee Beilstein-Institut.
Figures
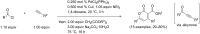


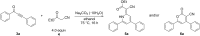

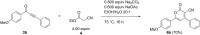
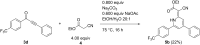
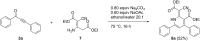
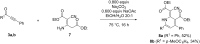





Similar articles
-
Fluorescent Donor-Acceptor Psoralen Cruciforms by Consecutive Suzuki-Suzuki and Sonogashira-Sonogashira One-Pot Syntheses.J Org Chem. 2020 Aug 7;85(15):9737-9750. doi: 10.1021/acs.joc.0c01059. Epub 2020 Jul 7. J Org Chem. 2020. PMID: 32575986
-
Three-Component Activation/Alkynylation/Cyclocondensation (AACC) Synthesis of Enhanced Emission Solvatochromic 3-Ethynylquinoxalines.Chemistry. 2018 Jun 7;24(32):8114-8125. doi: 10.1002/chem.201800079. Epub 2018 Mar 30. Chemistry. 2018. PMID: 29425410
-
1,2,5-Triphenylpyrrole Derivatives with Dual Intense Photoluminescence in Both Solution and the Solid State: Solvatochromism and Polymorphic Luminescence Properties.Chemistry. 2019 Jan 7;25(2):573-581. doi: 10.1002/chem.201804074. Epub 2018 Dec 13. Chemistry. 2019. PMID: 30357937
-
Electronic Finetuning of 8-Methoxy Psoralens by Palladium-Catalyzed Coupling: Acidochromicity and Solvatochromicity.Chemistry. 2020 Jun 26;26(36):8064-8075. doi: 10.1002/chem.201905676. Epub 2020 May 29. Chemistry. 2020. PMID: 32048795 Free PMC article.
-
One-Pot Coupling-Coupling-Cyclocondensation Synthesis of Fluorescent Pyrazoles.J Org Chem. 2016 Nov 4;81(21):10328-10338. doi: 10.1021/acs.joc.6b01326. Epub 2016 Jul 27. J Org Chem. 2016. PMID: 27403574
References
LinkOut - more resources
Full Text Sources
