Nitrogen-vacancy centers in diamond for nanoscale magnetic resonance imaging applications
- PMID: 31807400
- PMCID: PMC6880812
- DOI: 10.3762/bjnano.10.207
Nitrogen-vacancy centers in diamond for nanoscale magnetic resonance imaging applications
Abstract
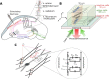
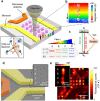
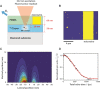
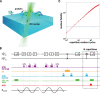
The nitrogen-vacancy (NV) center is a point defect in diamond with unique properties for use in ultra-sensitive, high-resolution magnetometry. One of the most interesting and challenging applications is nanoscale magnetic resonance imaging (nano-MRI). While many review papers have covered other NV centers in diamond applications, there is no survey targeting the specific development of nano-MRI devices based on NV centers in diamond. Several different nano-MRI methods based on NV centers have been proposed with the goal of improving the spatial and temporal resolution, but without any coordinated effort. After summarizing the main NV magnetic imaging methods, this review presents a survey of the latest advances in NV center nano-MRI.
Keywords: nanodiamonds; nanoscale magnetic resonance imaging (nano-MRI); nitrogen-vacancy center; optically detected magnetic resonance.
Copyright © 2019, Boretti et al.; licensee Beilstein-Institut.
Figures


Similar articles
-
Relaxometry with Nitrogen Vacancy (NV) Centers in Diamond.Acc Chem Res. 2022 Dec 20;55(24):3572-3580. doi: 10.1021/acs.accounts.2c00520. Epub 2022 Dec 7. Acc Chem Res. 2022. PMID: 36475573 Free PMC article. Review.
-
Calibration-Free Vector Magnetometry Using Nitrogen-Vacancy Center in Diamond Integrated with Optical Vortex Beam.Nano Lett. 2020 Nov 11;20(11):8267-8272. doi: 10.1021/acs.nanolett.0c03377. Epub 2020 Nov 2. Nano Lett. 2020. PMID: 33135901
-
Nitrogen-vacancy-assisted magnetometry of paramagnetic centers in an individual diamond nanocrystal.Nano Lett. 2012 Jul 11;12(7):3477-82. doi: 10.1021/nl300964g. Epub 2012 Jun 28. Nano Lett. 2012. PMID: 22725686
-
Advances in Stabilization and Enrichment of Shallow Nitrogen-Vacancy Centers in Diamond for Biosensing and Spin-Polarization Transfer.Biosensors (Basel). 2023 Jun 29;13(7):691. doi: 10.3390/bios13070691. Biosensors (Basel). 2023. PMID: 37504090 Free PMC article. Review.
-
Nanoscale quantum sensing with Nitrogen-Vacancy centers in nanodiamonds - A magnetic resonance perspective.Prog Nucl Magn Reson Spectrosc. 2023 Apr-Jun;134-135:20-38. doi: 10.1016/j.pnmrs.2022.12.001. Epub 2022 Dec 13. Prog Nucl Magn Reson Spectrosc. 2023. PMID: 37321756 Review.
Cited by
-
Long-Lived Ensembles of Shallow NV- Centers in Flat and Nanostructured Diamonds by Photoconversion.ACS Appl Mater Interfaces. 2021 Sep 15;13(36):43221-43232. doi: 10.1021/acsami.1c09825. Epub 2021 Sep 1. ACS Appl Mater Interfaces. 2021. PMID: 34468122 Free PMC article.
-
Cell-particles interaction - selective uptake and transport of microdiamonds.Commun Biol. 2024 Mar 13;7(1):318. doi: 10.1038/s42003-024-05974-4. Commun Biol. 2024. PMID: 38480800 Free PMC article.
-
Relaxometry with Nitrogen Vacancy (NV) Centers in Diamond.Acc Chem Res. 2022 Dec 20;55(24):3572-3580. doi: 10.1021/acs.accounts.2c00520. Epub 2022 Dec 7. Acc Chem Res. 2022. PMID: 36475573 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
