Phosphorylation of Tet3 by cdk5 is critical for robust activation of BRN2 during neuronal differentiation
- PMID: 31807777
- PMCID: PMC7026633
- DOI: 10.1093/nar/gkz1144
Phosphorylation of Tet3 by cdk5 is critical for robust activation of BRN2 during neuronal differentiation
Abstract
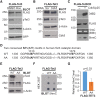
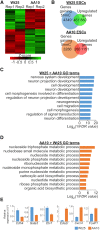
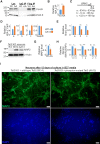
Tet3 regulates the dynamic balance between 5-methylcyotsine (5mC) and 5-hydroxymethylcytosine (5hmC) in DNA during brain development and homeostasis. However, it remains unclear how its functions are modulated in a context-dependent manner during neuronal differentiation. Here, we show that cyclin-dependent kinase 5 (cdk5) phosphorylates Tet3 at the highly conserved serine 1310 and 1379 residues within its catalytic domain, changing its in vitro dioxygenase activity. Interestingly, when stably expressed in Tet1, 2, 3 triple-knockout mouse embryonic stem cells (ESCs), wild-type Tet3 induces higher level of 5hmC and concomitant expression of genes associated with neurogenesis whereas phosphor-mutant (S1310A/S1379A) Tet3 causes elevated 5hmC and expression of genes that are linked to metabolic processes. Consistent with this observation, Tet3-knockout mouse ESCs rescued with wild-type Tet3 have higher level of 5hmC at the promoter of neuron-specific gene BRN2 when compared to cells that expressed phosphor-mutant Tet3. Wild-type and phosphor-mutant Tet3 also exhibit differential binding affinity to histone variant H2A.Z. The differential 5hmC enrichment and H2A.Z occupancy at BRN2 promoter is correlated with higher gene expression and more efficient neuronal differentiation of ESCs that expressed wild-type Tet3. Taken together, our results suggest that cdk5-mediated phosphorylation of Tet3 is required for robust activation of neuronal differentiation program.
© The Author(s) 2019. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

