Corticosteroids for treating sepsis in children and adults
- PMID: 31808551
- PMCID: PMC6953403
- DOI: 10.1002/14651858.CD002243.pub4
Corticosteroids for treating sepsis in children and adults
Update in
-
Corticosteroids for treating sepsis in children and adults.Cochrane Database Syst Rev. 2025 Jun 5;6(6):CD002243. doi: 10.1002/14651858.CD002243.pub5. Cochrane Database Syst Rev. 2025. PMID: 40470636
Abstract
Background: Sepsis occurs when an infection is complicated by organ failure. Sepsis may be complicated by impaired corticosteroid metabolism. Thus, providing corticosteroids may benefit patients. The original review was published in 2004 and was updated in 2010 and 2015 prior to this update.
Objectives: To examine the effects of corticosteroids on death in children and adults with sepsis.
Search methods: We searched CENTRAL, MEDLINE, Embase, LILACS, ClinicalTrials.gov, ISRCTN, and the WHO Clinical Trials Search Portal, on 25 July 2019. In addition, we conducted reference checking and citation searching, and contacted study authors, to identify additional studies as needed.
Selection criteria: We included randomized controlled trials (RCTs) of corticosteroids versus placebo or usual care (antimicrobials, fluid replacement, and vasopressor therapy as needed) in children and adults with sepsis. We also included RCTs of continuous infusion versus intermittent bolus of corticosteroids.
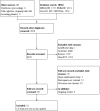
Data collection and analysis: All review authors screened and selected studies for inclusion. One review author extracted data, which was checked by the others, and by the lead author of the primary study when possible. We obtained unpublished data from the authors of some trials. We assessed the methodological quality of trials and applied GRADE to assess the certainty of evidence. Review authors did not contribute to assessment of eligibility and risk of bias, nor to data extraction, for trials they had participated in.
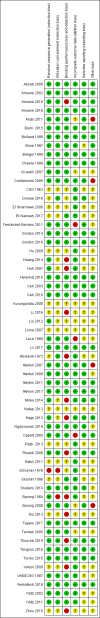
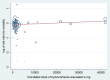
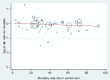
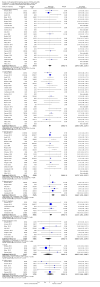
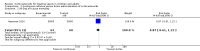
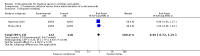
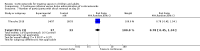
Main results: We included 61 trials (12,192 participants), of which six included only children, two included children and adults, and the remaining trials included only adults. Nine studies are ongoing and will be considered in future versions of this review. We judged 19 trials as being at low risk of bias. Corticosteroids versus placebo or usual care Compared to placebo or usual care, corticosteroids probably slightly reduce 28-day mortality (risk ratio (RR) 0.91, 95% confidence interval (CI) 0.84 to 0.99; 11,233 participants; 50 studies; moderate-certainty evidence). Corticosteroids may result in little to no difference in long-term mortality (RR 0.97, 95% CI 0.91 to 1.03; 6236 participants; 7 studies; low-certainty evidence) and probably slightly reduce hospital mortality (RR 0.90, 95% CI 0.82 to 0.99; 8183 participants; 26 trials; moderate-certainty evidence). Corticosteroids reduced length of intensive care unit (ICU) stay for all participants (mean difference (MD) -1.07 days, 95% CI -1.95 to -0.19; 7612 participants; 21 studies; high-certainty evidence) and resulted in a large reduction in length of hospital stay for all participants (MD -1.63 days, 95% CI -2.93 to -0.33; 8795 participants; 22 studies; high-certainty evidence). Corticosteroids increase the risk of muscle weakness (RR 1.21, 95% CI 1.01 to 1.44; 6145 participants; 6 studies; high-certainty evidence). Corticosteroids probably do not increase the risk of superinfection (RR 1.06, 95% CI 0.95 to 1.19; 5356 participants; 25 studies; moderate-certainty evidence). Corticosteroids increase the risk of hypernatraemia (high-certainty evidence) and probably increase the risk of hyperglycaemia (moderate-certainty evidence). Moderate-certainty evidence shows that there is probably little or no difference in gastroduodenal bleeding, stroke, or cardiac events, and low-certainty evidence suggests that corticosteroids may result in little to no difference in neuropsychiatric events. Continuous infusion of corticosteroids versus intermittent bolus We are uncertain about the effects of continuous infusion of corticosteroids compared with intermittent bolus administration. Three studies reported data for this comparison, and the certainty of evidence for all outcomes was very low.
Authors' conclusions: Moderate-certainty evidence indicates that corticosteroids probably reduce 28-day and hospital mortality among patients with sepsis. Corticosteroids result in large reductions in ICU and hospital length of stay (high-certainty evidence). There may be little or no difference in the risk of major complications; however, corticosteroids increase the risk of muscle weakness and hypernatraemia, and probably increase the risk of hyperglycaemia. The effects of continuous versus intermittent bolus administration of corticosteroids are uncertain.
Copyright © 2019 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
Djillali Annane is an author of the following studies included in this review: Aboab 2008; Annane 2002; Annane 2010; Annane 2018; Sprung 2008. He obtained funds from the French Minisitry of Health to conduct the following trials: Annane 2002; Annane 2010; and Annane 2018. He has been the chair of the international task force for elaborating 2017 guidelines for the diagnosis and treatment of critical illness‐related corticosteroid insufficiency.
Eric Bellissant is an author of the following studies included in this review: Annane 2002; Annane 2018.
Pierre Edouard Bollaert is an author of the following studies included in this review: Annane 2002; Bollaert 1998. He obtained public funds from the University of Nancy to conduct the trial (Bollaert 1998).
Josef Briegel is an author of the following studies included in this review: Briegel 1999; Keh 2016; Sprung 2008. He obtained public funds to conduct the trial (Briegel 1999). He contributed to the international task force for elaborating 2017 guidelines for the diagnosis and treatment of critical illness‐related corticosteroid insufficiency. He participated in the European Society of Intensive Care Medicine, the Deutsche interdisziplinäre Vereinigung Intensivmedizin, and the Deutsche Gesellschaft für Anästhesie und Intensivmedizin, and he has given lectures and talks on hydrocortisone treatment for septic shock.
Didier Keh is an author of the following studies included in this review: Keh 2003; Keh 2016; Sprung 2008. He obtained public funds from Charité–Universitätsmedizin Berlin and from the German Federal Ministry of Education and Research to conduct the following trials: Keh 2003; Keh 2016.
Yizhak Kupfer is an author of the following studies included in this review: Chawla 1999. He is a member of the Pfizer/BMS speakers' bureau for epixaban. This product has no relationship to steroids in sepsis. He obtained funds from his institution to conduct the trial (Chawla 1999).
Romain Pirracchio received funding for International Mobility from the Fulbright Foundation and from the Assistance Publique – Hôpitaux de Paris (APHP).
Bram Rochwerg is supported by McMaster University Department of Medicine early career research awards. He has contributed to the international task force for elaborating 2017 guidelines for the diagnosis and treatment of critical illness‐related corticosteroid insufficiency. He is a methodologist for American Thoracic Society, European Society of Intensive Care Medicine, and American Society of Haematology.
Figures






















Update of
-
Corticosteroids for treating sepsis.Cochrane Database Syst Rev. 2015 Dec 3;2015(12):CD002243. doi: 10.1002/14651858.CD002243.pub3. Cochrane Database Syst Rev. 2015. Update in: Cochrane Database Syst Rev. 2019 Dec 6;12:CD002243. doi: 10.1002/14651858.CD002243.pub4. PMID: 26633262 Free PMC article. Updated.
Comment in
-
Corticosteroids probably reduce sepsis-related 28-day mortality in adults - unclear effect in children.J Pediatr. 2020 May;220:264-267. doi: 10.1016/j.jpeds.2020.02.067. J Pediatr. 2020. PMID: 32334669 No abstract available.
References
References to studies included in this review
Aboab 2008 {published data only (unpublished sought but not used)}
-
- Aboab J, Polito A, Orlikowski D, Sharshar T, Castel M, Annane D. Hydrocortisone effects on cardiovascular variability in septic shock: a spectral analysis approach. Critical Care Medicine 2008;36(5):1481‐6. [PUBMED: 18434902] - PubMed
Annane 2002 {published and unpublished data}
-
- Annane D, Sebille V, Charpentier C, Bollaert PE, François B, Korach JM, et al. Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. JAMA 2002;288(7):862‐71. [PUBMED: 12186604] - PubMed
Annane 2010 {published and unpublished data}
-
- COIITSS Study Investigators, Annane D, Cariou A, Maxime V, Azoulay E, D'honneur G, et al. Corticosteroid treatment and intensive insulin therapy for septic shock in adults: a randomized controlled trial. JAMA 2010;303(4):341‐8. [PUBMED: 20103758] - PubMed
Annane 2018 {published and unpublished data}
-
- Annane D, Renault A, Brun‐Buisson C, Megarbane B, Quenot JP, Siami S, et al. Hydrocortisone plus fludrocortisone for adults with septic shock. New England Journal of Medicine 2018;378(9):809‐18. [PUBMED: 29490185] - PubMed
Arabi 2011 {published and unpublished data}
Blum 2015 {published data only}
-
- Blum CA, Nigro N, Briel M, Schuetz P, Ullmer E, Suter‐Widmer I, et al. Adjunct prednisone therapy for patients with community‐acquired pneumonia: a multicentre, double‐blind, randomised, placebo‐controlled trial. Lancet 2015;385(9977):1511‐8. [PUBMED: 25608756] - PubMed
Bollaert 1998 {published and unpublished data}
-
- Bollaert PE, Charpentier C, Levy B, Debouverie M, Audibert G, Larcan A. Reversal of late septic shock with supraphysiologic doses of hydrocortisone. Critical Care Medicine 1998;26(4):645‐50. [PUBMED: 9559600] - PubMed
Bone 1987 {published data only}
-
- Bone RG, Fisher CJ, Clemmer TP, Slotman GJ, Metz CA, Balk RA. A controlled clinical trial of high‐dose methylprednisolone in the treatment of severe sepsis and septic shock. New England Journal of Medicine 1987;317(11):653‐8. [PUBMED: 3306374] - PubMed
Briegel 1999 {published and unpublished data}
-
- Briegel J, Forst H, Haller M, Schelling G, Kilger E, Kuprat G, et al. Stress doses of hydrocortisone reverse hyperdynamic septic shock: a prospective, randomized, double‐blind, single‐center study. Critical Care Medicine 1999;27(4):723‐32. [PUBMED: 10321661] - PubMed
Chawla 1999 {published and unpublished data}
-
- Chawla K, Kupfer Y, Tessler S. Hydrocortisone reverses refractory septic shock. Critical Care Medicine 1999;27(1):A33.
Cicarelli 2007 {published data only}
Confalonieri 2005 {published and unpublished data}
-
- Confalonieri M, Urbino R, Potena A, Piatella M, Parigi P, Giacomo P, et al. Hydrocortisone infusion for severe community acquired pneumonia: a preliminary randomized study. American Journal of Respiratory and Critical Care Medicine 2005;171(3):242‐8. [PUBMED: 15557131] - PubMed
CSG 1963 {published data only}
-
- Cooperative Study Group. The effectiveness of hydrocortisone in the management of severe infections. JAMA 1963;183(6):462‐5. [DOI: 10.1001/jama.1963.63700060029012] - DOI
Doluee 2018 {published data only}
-
- Talebi Doluee M, Salehi M, Mahmoudi Gharaee A, Jalalyazdi M, Reihani H. The effect of physiologic dose of intravenous hydrocortisone in patients with refractory septic shock: a randomized control trial. Journal of Emergency Practice and Trauma 2018;4:29‐33. [DOI: 10.15171/JEPT.2017.25] - DOI
El Ghamrawy 2006 {published data only}
-
- El‐Ghamraway AH, Shokeir MH, Esmat AA. Effects of low dose hydrocortisone in ICU patients with severe community acquired pneumonia. Egyptian Journal of Chest 2006;55:91‐9.
El‐Nawawy 2017 {published data only}
-
- El‐Nawawy A, Khater D, Omar H, Wali Y. Evaluation of early corticosteroid therapy in management of paediatric septic shock in paediatric intensive care patients: a randomized clinical study. Pediatric Infectious Disease Journal 2017;36(2):155‐9. [PUBMED: 27798546] - PubMed
Fernández‐Serrano 2011 {published data only}
Gordon 2014 {published and unpublished data}
-
- Gordon AC, Mason AJ, Perkins GD, Stotz M, Terblanche M, Ashby D, et al. The interaction of vasopressin and corticosteroids in septic shock: a pilot randomized controlled trial. Critical Care Medicine 2014;42(6):1325‐33. [PUBMED: 24557425] - PubMed
Gordon 2016 {published and unpublished data}
-
- Gordon AC, Mason AJ, Thirunavukkarasu N, Perkins GD, Cecconi M, Cepkova M, et al. Effect of early vasopressin vs norepinephrine on kidney failure in patients with septic shock: the VANISH randomized clinical trial. JAMA 2016;316(5):509‐18. [PUBMED: 27483065] - PubMed
Hu 2009 {published data only (unpublished sought but not used)}
-
- Hu B, Li JG, Liang H, Zhou Q, Yu Z, Li L, et al. The effect of low‐dose hydrocortisone on requirement of norepinephrine and lactate clearance in patients with refractory septic shock. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue 2009;21(9):529‐31. [PUBMED: 19751560] - PubMed
Huang 2014 {published data only}
-
- Huang R, Zhang Z, Xu M, Chang X, Qiao Q, Wang L, et al. Effect of Sini decoction on function of hypothalamic‐pituitary‐adrenal axis in patients with sepsis. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2014;26(3):184‐7. [PUBMED: 24598293] - PubMed
Huh 2007 {published data only}
-
- Huh JW, Lim CM, Koh Y, Hong SB. Effect of low doses of hydrocortisone in patient with septic shock and relative adrenal insufficiency: 3 days versus 7 days treatment. Critical Care Medicine 2006;34(12 suppl):A101.
Hyvernat 2016 {published and unpublished data}
-
- Hyvernat H, Barel R, Gentilhomme A, Césari‐Giordani JF, Freche A, Kaidomar M, et al. Effects of increasing hydrocortisone to 300 mg per day in the treatment of septic shock: a pilot study. Shock 2016;46:498‐505. [PUBMED: 27405061] - PubMed
Keh 2003 {published and unpublished data}
-
- Keh D, Boehnke T, Weber‐Cartens S, Schulz C, Ahlers O, Bercker S, et al. Immunologic and hemodynamic effects of "low‐dose" hydrocortisone in septic shock: a double‐blind, randomized, placebo‐controlled, crossover study. American Journal of Respiratory and Critical Care Medicine 2003;167(4):512‐20. [PUBMED: 12426230 ] - PubMed
Keh 2016 {published and unpublished data}
-
- Keh D, Trips E, Marx G, Wirtz SP, Abduljawwad E, Bercker S, et al. Effect of hydrocortisone on development of shock among patients with severe sepsis: the HYPRESS randomized clinical trial. JAMA 2016;316(17):1775‐85. [PUBMED: 27695824] - PubMed
Kurungundla 2008 {published data only (unpublished sought but not used)}
-
- Kurugundla N, Irugulapati L, Kilari D, Amchentsev A, Devakonda A, George L, et al. Effect of steroids in septic shock patients without relative adrenal insufficiency. American Thoracic Society 2008;116.
Li 2016 {published data only}
-
- Li G, Gu C, Zhang S, Lian R, Zhang G. Value of glucocorticoid steroids in the treatment of patients with severe community acquired pneumonia complicated with septic shock. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2016;28(9):780‐4. [DOI: 10.3760/cma.j.issn.2095-4352.2016.09.003] - DOI
Liu 2012 {published and unpublished data}
-
- Liu L, Li J, Huang YZ, Liu SQ, Yang CS, Guo FM, et al. The effect of stress dose glucocorticoid on patients with acute respiratory distress syndrome combined with critical illness‐related corticosteroid insufficiency. Zhonghua Nei Ke Za Zhi 2012;51(8):599‐603. [PUBMED: 23158856] - PubMed
Loisa 2007 {published data only}
Luce 1988 {published and unpublished data}
-
- Luce JM, Montgomery AB, Marks JD, Turner J, Metz CA, Murray JF. Ineffectiveness of high‐dose methylprednisolone in preventing parenchymal lung injury and improving mortality in patients with septic shock. American Review of Respiratory Diseases 1988;138(1):62‐8. [PUBMED: 3202402 ] - PubMed
Lv 2017 {published and unpublished data}
-
- Lv QQ, Gu XH, Chen QH, Yu JQ, Zheng RQ. Early initiation of low‐dose hydrocortisone treatment for septic shock in adults: a randomized clinical trial. American Journal of Emergency Medicine 2017;35(12):1810‐4. [PUBMED: 28615145] - PubMed
McHardy 1972 {published data only}
Meduri 2007 {published and unpublished data}
-
- Meduri GU, Golden E, Freire AX, Taylor E, Zaman M, Carson SJ, et al. Methylprednisolone infusion in early severe ARDS: results of a randomized controlled trial. Chest 2007;131(4):954‐63. [PUBMED: 17426195] - PubMed
Meduri 2009 {published data only (unpublished sought but not used)}
-
- Meduri GU, Golden E, Freire AX, Umberger R. Randomized clinical trial (RCT) evaluating the effects of low‐dose prolonged hydrocortisone infusion on resolution of MODS in severe sepsis. Chest 2009;136:154.
Meijvis 2011 {published data only}
-
- Meijvis SC, Hardeman H, Remmelts HH, Heijligenberg R, Rijkers GT, Velzen‐Blad H, et al. Dexamethasone and length of hospital stay in patients with community‐acquired pneumonia: a randomised, double‐blind, placebo‐controlled trial. Lancet 2011;377(9782):2023‐30. [PUBMED: 21636122] - PubMed
Menon 2017 {published data only}
Mirea 2014 {published and unpublished data}
-
- Mirea L, Ungureanu R, Pavelescu D, Grintescu IC, Dumitrache C, Grintescu I, et al. Continuous administration of corticosteroids in septic shock can reduce risk of hypernatraemia. Critical Care 2014;18:S86.
Nafae 2013 {published data only}
-
- Nafae RM, Ragab MI, Amany FM, Rashed SB. Adjuvant role of corticosteroids in the treatment of community acquired pneumonia. Egyptian Journal of Chest Diseases and Tuberculosis 2013;62:439‐45.
Nagy 2013 {published data only}
-
- Nagy B, Gaspar I, Papp A, Bene Z, Nagy B Jr, Voko Z, et al. Efficacy of methylprednisolone in children with severe community acquired pneumonia. Pediatric Pulmonology 2013;48:168‐75. [PUBMED: 22588852] - PubMed
Ngaosuwan 2018 {published data only}
-
- Ngaosuwan K, Ounchokdee K, Chalermchai T. Clinical outcomes of minimized hydrocortisone dosage of 100mg/day on lower occurrence of hyperglycaemia in septic shock. Shock 2018;50(3):280‐5. [PUBMED: 29176402] - PubMed
Oppert 2005 {published and unpublished data}
-
- Oppert M, Schindler R, Husung C, Offerman K, Graef KJ, Boenisch O, et al. Low‐dose hydrocortisone improves shock reversal and reduces cytokine levels in early hyperdynamic septic shock. Critical Care Medicine 2005;33(11):2457‐64. [PUBMED: 16276166 ] - PubMed
Rezk 2013 {published data only}
-
- Rezk NA, Ibrahim AM. Effects of methyl prednisolone in early ARDS. Egyptian Journal of Chest Diseases and Tuberculosis 2013;62(1):167–72. [DOI: 10.1016/j.ejcdt.2013.02.013] - DOI
Rinaldi 2006 {published data only}
-
- Rinaldi S, Adembri C, Grechi S, DeGaudio R. Low‐dose hydrocortisone during severe sepsis: effects on microalbuminemia. Critical Care Medicine 2006;34(9):2334‐9. [PUBMED: 16850006] - PubMed
Sabry 2011 {published data only}
-
- Sabry NA, El‐Din Omar E. Corticosteroids and ICU course of community acquired pneumonia in Egyptian settings. Pharmacology and Pharmacy 2011;2:73‐81.
Schumer 1976 {published data only}
Slusher 1996 {published data only}
-
- Slusher T, Gbadero D, Howard C, Lewinson L, Giroir B, Toro L, et al. Randomized, placebo‐controlled, double blinded trial of dexamethasone in African children with sepsis. Pediatric Infectious Diseases Journal 1996;15(7):579‐83. [PUBMED: 8823850] - PubMed
Snijders 2010 {published data only}
-
- Snijders D, Daniels JMA, Graaff CS, Werf TS, Boersma WG. Efficacy of corticosteroids in community‐acquired pneumonia – a randomized double blinded clinical trial. American Journal of Respiratory and Critical Care Medicine 2010;181(9):975‐82. [PUBMED: 20133929] - PubMed
Sprung 1984 {published and unpublished data}
-
- Sprung CL, Caralis PV, Marcial EH, Pierce M, Gelbard MA, Long WM, et al. The effects of high‐dose corticosteroids in patients with septic shock. A prospective, controlled study. New England Journal of Medicine 1984;311(18):1137‐43. [PUBMED: 6384785 ] - PubMed
Sprung 2008 {published and unpublished data}
-
- Sprung C, Annane D, Keh D, Moreno R, Singer M, Freivogel K, et al. Hydrocortisone therapy for patients with septic shock. New England Journal of Medicine 2008;358(2):111‐24. [PUBMED: 18184957] - PubMed
Sui 2013 {published data only}
-
- Sui DJ, Zhang W, Li WS, Zhao HX, Wang ZY. Clinical efficacy in the treatment of severe community acquired pneumonia and its impact on CRP. Chinese Journal of Integrative Medicine 2013;18:1171‐3.
Tagaro 2017 {published data only}
-
- Tagaro A, Otheo E, Baquero‐Artigao F, Navarro ML, Velasco R, Ruiz M, et al. Dexamethasone for parapneumonic pleural effusion: a randomized, double‐blind, clinical trial. Journal of Pediatrics 2017;185:117‐23. [PUBMED: 28363363] - PubMed
Tandan 2005 {unpublished data only}
-
- Tandan SM, Guleria R, Gupta N. Low dose steroids and adrenocortical insufficiency in septic shock: a double‐blind randomised controlled trial from India. Proceedings of the American Thoracic Society Meeting. 2005:A24.
Tilouche 2019 {published data only}
-
- Tilouche N, Jaoued O, Ben Sik Ali H, Gharbi R, Fekih Hassen M, Elatrous S. Comparison between continuous and intermittent administration of hydrocortisone during septic shock: a randomized controlled clinical trial. Shock 2019;52:481‐6. [DOI: 10.1097/SHK.0000000000001316.; PUBMED: 30628950] - DOI - PubMed
Tongyoo 2016 {published and unpublished data}
Torres 2015 {published data only}
-
- Torres A, Sibila O, Ferrer M, Polverino E, Menendez R, Mensa J, et al. Effect of corticosteroids on treatment failure among hospitalized patients with severe community‐acquired pneumonia and high inflammatory response: a randomized clinical trial. JAMA. 2015; Vol. 313, issue 7:677‐86. [PUBMED: 25688779] - PubMed
Valoor 2009 {published data only}
-
- Valoor HT, Singhi S, Jayashree M. Low‐dose hydrocortisone in paediatric septic shock: an exploratory study in a third world setting. Pediatric Critical Care Medicine 2009;10(1):121‐5. [PUBMED: 19057445] - PubMed
VASSCSG 1987 {published data only}
-
- Veterans Administration Systemic Sepsis Cooperative Study Group. Effect of high‐dose glucocorticoid therapy on mortality in patients with clinical signs of systemic sepsis. New England Journal of Medicine 1987;317(11):659‐65. [PUBMED: 2888017] - PubMed
Venkatesh 2018 {published data only (unpublished sought but not used)}
-
- Venkatesh B, Finfer S, Mybrugh J, Cohen J, Billot L. Long‐term outcomes of the ADRENAL trial [2018]. New England Journal of Medicine 2018;378(18):174‐5. [PUBMED: 29694789] - PubMed
Yildiz 2002 {published data only}
Yildiz 2011 {published data only}
Zhou 2015 {published data only}
-
- Zhou M. Application value of glucocorticoid for comprehensive treatment of acute respiratory distress syndrome induced by serious community acquired pneumonia. Clinical Medicine and Engineering 2015;22:57‐8. [DOI: 10.3969/j.issn.1674-4659.2015.01.0057] - DOI
References to studies excluded from this review
Asehnoune 2014 {published data only}
-
- Asehnoune K, Seguin P, Allary J, Feuillet F, Lasocki S, Cook F, et al. Hydrocortisone and fludrocortisone for prevention of hospital‐acquired pneumonia in patients with severe traumatic brain injury (Corti‐TC): a double‐blind, multicentre phase 3, randomised placebo‐controlled trial. The Lancet. Respiratory Medicine 2014;2:706‐16. [PUBMED: 25066331] - PubMed
Bernard 1987 {published data only}
-
- Bernard GR, Luce JM, Sprung CL, Rinaldo JE, Tate RM, Sibbald WJ, et al. High‐dose corticosteroids in patients with the adult respiratory distress syndrome. New England Journal of Medicine 1987;317:1565‐70. [PUBMED: 3317054] - PubMed
Cicarelli 2006 {published data only}
Hahn 1951 {published data only}
Huang 2014a {published data only}
-
- Huang L, Gao X, Chen M. Early treatment with corticosteroids in patients with Mycoplasma pneumoniae pneumonia: a randomized clinical trial. Journal of Tropical Pediatrics 2014;64:338‐42. [PUBMED: 24710342] - PubMed
Huang 2015 {published data only}
-
- Huang G, Liang B, Liu G, Liu K, Ding Z. Low dose of glucocorticoid decreases the incidence of complications in severely burned patients by attenuating systemic inflammation. J Crit Care 2015;436:436.e7‐11. - PubMed
Hughes 1984 {published data only}
-
- Hughes GS Jr. Naloxone and methylprednisolone sodium succinate enhance sympathomedullary discharge in patients with septic shock. Life Sciences 1984;35(23):2319‐26. [PUBMED: 6390057] - PubMed
Kaufman 2008 {published and unpublished data}
-
- Kaufmann I, Briegel J, Schliephake F, Hoelzl A, Chouker A, Hummel T, et al. Stress doses of hydrocortisone in septic shock: beneficial effects on opsonization‐dependent neutrophil functions. Intensive Care Medicine 2008;34(2):344‐9. [PUBMED: 17906853 ] - PubMed
Klastersky 1971 {published data only}
-
- Klastersky J, Cappel R, Debusscher L. Effectiveness of betamethasone in management of severe infections. A double‐blind study. New England Journal of Medicine 1971;284(22):1248‐50. [PUBMED: 4929896] - PubMed
Lan 2015 {published data only}
-
- Lan Y, Yang D, Chen Z, Tang L, Xu Y, Cheng Y. Effectiveness of methylprednisolone in treatment of children with refractory Mycoplasma pneumoniae pneumonia and its relationship with bronchoalveolar lavage cytokine levels. Zhonghua Er Ke Za Zhi 2015;53:779‐83.. - PubMed
Lucas 1984 {published data only}
-
- Lucas C, Ledgerwood A. The cardiopulmonary response to massive doses of steroids in patients with septic shock. Archives of Surgery 1984;119(5):537‐41. [PUBMED: 6712466 ] - PubMed
Luo 2014 {published data only}
-
- Luo Z, Luo J, Liu E, Xu X, Liu Y, Zeng F, et al. Effects of prednisolone on refractory Mycoplasma pneumoniae pneumonia in children. Pediatric Pulmonology 2014;49:377‐80. [PUBMED: 23401275] - PubMed
Marik 1993 {published data only}
-
- Marik P, Kraus P, Sribante J, Havlik I, Lipman J, Johnson DW. Hydrocortisone and tumour necrosis factor in severe community‐acquired pneumonia. A randomized controlled study. Chest 1993;104:389‐92. [PUBMED: 8339624] - PubMed
McKee 1983 {published data only}
-
- McKee JI, Finlay WE. Cortisol replacement in severely stressed patients. Lancet 1983;1(8322):484. [PUBMED: 6131207 ] - PubMed
Meduri 1998b {published and unpublished data}
-
- Meduri GU, Headley AS, Golden E, Carson SJ, Umberger RA, Kelso T, et al. Effect of prolonged methylprednisolone therapy in unresolving acute respiratory distress syndrome: a randomized controlled trial. JAMA 1998;280(2):159‐65. [PUBMED: 9669790] - PubMed
Mikami 2007 {published data only}
-
- Mikami K, Suzuki M, Kitagawa H, Kawakami M, Hirota N, Yamaguchi H, et al. Efficacy of corticosteroids in the treatment of community‐acquired pneumonia requiring hospitalization. Lung 2007;185(5):249‐55. [PUBMED: 17710485] - PubMed
Newberry 2017 {published data only}
-
- Newberry L, O'Hare B, Kennedy N, Selman A, Omar S, Dawson P, Stevenson K, Nishihara Y, Lissauer S, Molyneux E. Early use of corticosteroids in infants with a clinical diagnosis of Pneumocystis jiroveci pneumonia in Malawi: a double‐blind, randomised clinical trial. Paediatr Int Child Health. 2017;37:121‐128.. - PubMed
Peeters 2018 {published data only}
-
- Peeters B, Meersseman P, Vander Perre S, Wouters PJ, Debaveye Y, Langouche L, Berghe G. ACTH and cortisol responses to CRH in acute, subacute, and prolonged critical illness: a randomized, double‐blind, placebo‐controlled, crossover cohort study. Intensive Care Medicine 2018;44:2048‐58.. - PMC - PubMed
Rogers 1970 {published data only}
-
- Rogers J. Large doses of steroids in septicaemic shock. British Journal of Urology 1970;42(6):742. [PUBMED: 4923652] - PubMed
Roquilly 2011 {published data only}
-
- Roquilly A, Mahe PJ, Seguin P, Guitton C, Floch H, Tellier AC, et al. Hydrocortisone therapy for patients with multiple trauma: the randomized controlled HYPOLYTE study. JAMA 2011;305:1201‐9. [PUBMED: 21427372] - PubMed
Schwingshackl 2016 {published data only}
-
- Schwingshackl A, Kimura D, Rovnaghi CR, Saravia JS, Cormier SA, Teng B, et al. Regulation of inflammatory biomarkers by intravenous methylprednisolone in pediatric ARDS patients: results from a double‐blind, placebo‐controlled randomized pilot trial. Cytokine 2016;77:63‐71. [PUBMED: 26545141] - PMC - PubMed
Steinberg 2006 {published data only}
-
- Steinberg KP, Hudson LD, Goodman RB, Hough CL, Lanken PN, Hyzy R, et al. Efficacy and safety of corticosteroids for persistent acute respiratory distress syndrome. New England Journal of Medicine 2006;354:1671‐84. [PUBMED: 16625008] - PubMed
Tam 2012 {published data only}
Thompson 1976 {published data only}
-
- Thompson WL, Gurley HT, Lutz BA, Jackson DL, Kvols LK, Morris IA. Inefficacy of glucocorticoids in shock (double‐blind study). Clinical Research 1976;24:258A.
van Woensel 2003 {published data only}
Venet 2015 {published data only}
Wagner 1955 {published data only}
-
- Wagner HN, Bennett IL, Lasagna L, Cluff LE, Rosenthal MB, Mirick GS. The effect of hydrocortisone upon the course of pneumococcal pneumonia treated with penicillin. Bulletin of Johns Hopkins Hospital 1955;98(3):197‐215. [PUBMED: 13304518] - PubMed
Weigelt 1985 {published data only}
-
- Weigelt JA, Norcross JF, Borman KR, Snyder WH 3rd. Early steroid therapy for respiratory failure. Archives of Surgery 1985;120(5):536‐40. [PUBMED: 3885915 ] - PubMed
References to ongoing studies
NCT02517489 {published data only}
-
- NCT02517489. Community‐acquired pneumonia: evaluation of corticosteroids (CAPE COD) [Effects of low‐dose corticosteroids on survival of severe community‐acquired pneumonia]. https://clinicaltrials.gov/ct2/show/NCT02517489. First received 7 August 2015.
NCT02602210 {published data only}
-
- NCT02602210. Supplemental corticosteroids in cirrhotic hypotensive patients with suspicion of sepsis (SCOTCH) [Supplemental corticosteroids in cirrhotic hypotensive patients with suspicion of sepsis]. https://clinicaltrials.gov/ct2/show/NCT02602210. First received 11 November 2015.
NCT03258684 {published data only}
-
- NCT03258684. Hydrocortisone, vitamin C, and thiamine for the treatment of sepsis and septic shock (HYVCTTSSS) [Hydrocortisone, vitamin C, and thiamine for the treatment of sepsis and septic shock: a prospective study]. https://clinicaltrials.gov/ct2/show/NCT03258684. First received 23 August 2017.
NCT03333278 {published data only}
-
- NCT03333278. The vitamin C, hydrocortisone and thiamine in patients with septic shock trial (VITAMINS) [The vitamIn C, hydrocortisone and thiamine in patients with septic shock trial (VITAMINS trial) ‐ a prospective, feasibility, pilot, multi‐centre, randomised, open‐label controlled trial]. https://clinicaltrials.gov/ct2/show/NCT03333278. First received 6 November 2017.
NCT03335124 {published data only}
-
- NCT03335124. The effect of vitamin C, thiamine and hydrocortisone on clinical course and outcome in patients with severe sepsis and septic shock [A randomized, double blind, placebo‐controlled study to investigate the effects of vitamin C, hydrocortisone and thiamine on the outcome of patients with severe sepsis and septic shock]. https://clinicaltrials.gov/ct2/show/NCT03335124. First received 7 November 2017.
NCT03389555 {published data only}
-
- NCT03389555. Ascorbic acid, corticosteroids, and thiamine in sepsis (ACTS) trial [Ascorbic acid, hydrocortisone, and thiamine in sepsis and septic shock ‐ a randomized, double‐blind, placebo‐controlled trial]. https://clinicaltrials.gov/ct2/show/NCT03389555. First received 3 January 2018.
NCT03422159 {published data only}
-
- NCT03422159. Metabolic resuscitation using ascorbic acid, thiamine, and glucocorticoids in sepsis (ORANGES) [Outcomes of metabolic resuscitation using ascorbic acid, thiamine, and glucocorticoids in the early treatment of sepsis]. https://clinicaltrials.gov/ct2/show/NCT03422159. First received 5 February 2018.
NCT03509350 {published data only}
-
- NCT03509350. Vitamin C, thiamine, and steroids in sepsis (VICTAS) [A multi‐center, randomized, placebo‐controlled, double‐blind, adaptive clinical trial of vitamin C, thiamine and steroids as combination therapy in patients with sepsis]. https://clinicaltrials.gov/ct2/show/NCT03509350. First received 26 April 2018.
NCT03592693 {published data only}
-
- NCT03592693. Vitamin C, hydrocortisone and thiamine for septic shock (CORVICTES) [A randomized, double blind, placebo‐controlled trial to investigate the effect of vitamin C, hydrocortisone and thiamine on the outcome of patients with septic shock]. https://clinicaltrials.gov/ct2/show/NCT03592693. First received 19 July 2018.
Additional references
ACCP/SCCM 1992
-
- ACCP/SCCM Consensus Conference Panel. American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Critical Care Medicine 1992;20(6):864‐74. [PUBMED: 1597042 ] - PubMed
Allen 2018
-
- Allen JM, Feild C, Shoulders BR, Voils SA. Recent updates in the pharmacological management of sepsis and septic shock: a systematic review focused on fluid resuscitation, vasopressors, and corticosteroids. Annals of Pharmacotherapy 2018;53:385‐95. [DOI: 10.1177/1060028018812940; PUBMED: 30404539 ] - DOI - PubMed
Angus 2013
-
- Angus DC, Poll T. Severe sepsis and septic shock. New England Journal of Medicine 2013;369(21):840‐51. [PUBMED: 23984731] - PubMed
Annane 2000
-
- Annane D, Sébille V, Troché G, Raphael JC, Gajdos P, Bellissant E. A 3‐level prognostic classification in septic shock based on cortisol levels and cortisol response to corticotropin. JAMA 2000;283(8):1038‐45. [PUBMED: 10697064] - PubMed
Annane 2003
-
- Annane D, Aegerter P, Jars‐Guincestre MC, Guidet B, CUB‐Rea Network. Current epidemiology of septic shock: the CUB‐Réa Network. American Journal of Respiratory and Critical Care Medicine 2003;168(2):165‐72. [PUBMED: 12851245] - PubMed
Annane 2005
-
- Annane D, Bellissant E, Cavaillon JM. Septic shock. Lancet 2005;365(9453):63‐78. [PUBMED: 15639681] - PubMed
Annane 2009b
-
- Annane D. Improving clinical trials in the critically ill: unique challenge: sepsis. Critical Care Medicine 2009;37(1 Suppl):117‐28. [PUBMED: 19104211] - PubMed
Annane 2017a
-
- Annane D, Pastores SM, Arlt W, Balk RA, Beishuizen A, Briegel J, et al. Critical illness‐related corticosteroid insufficiency (CIRCI): a narrative review from a Multispecialty Task Force of the Society of Critical Care Medicine (SCCM) and the European Society of Intensive Care Medicine (ESICM). Intensive Care Medicine 2017;43(12):1781‐92. [PUBMED: 28940017] - PubMed
Annane 2017b
-
- Annane D, Pastores SM, Rochwerg B, Arlt W, Balk RA, Beishuizen A, et al. Guidelines for the diagnosis and management of critical illness‐related corticosteroid insufficiency (CIRCI) in critically ill patients (Part I): Society of Critical Care Medicine (SCCM) and European Society of Intensive Care Medicine (ESICM) 2017. Intensive Care Medicine 2017;43(12):1751‐63. [PUBMED: 28940011] - PubMed
Barczyk 2010
-
- Barczyk K, Ehrchen J, Tenbrock K, Ahlmann M, Kneidl J, Viemann D, et al. Glucocorticoids promote survival of anti‐inflammatory macrophages via stimulation of adenosine receptor A3. Blood 2010;116(3):446‐55. [PUBMED: 20460503] - PubMed
Barnes 1995
-
- Barnes PJ, Greening AP, Crompton GK. Glucocorticoid resistance in asthma. American Journal of Respiratory and Critical Care Medicine 1995;152(Suppl 6 Pt 2):125‐40. [PUBMED: 7489120] - PubMed
Boonen 2013
Briegel 1994
-
- Briegel J, Kellermann W, Forst H, Haller M, Bittl M, Hoffmann GE, et al. Low‐dose hydrocortisone infusion attenuates the systemic inflammatory response syndrome. The Phospholipase A2 Study Group. Clinical Investigation 1994;72(10):782‐7. [PUBMED: 7865982] - PubMed
Bruno 2012
-
- Bruno JJ, Dee BM, Anderegg BA, Hernandez M, Pravinkumar SE. US practitioner opinions and prescribing practices regarding corticosteroid therapy for severe sepsis and septic shock. Journal of Critical Care 2012;27(4):351‐61. [PUBMED: 22341726] - PubMed
Cai 2008
Cain 2017
Chrousos 1995
-
- Chrousos GP. The hypothalamic‐pituitary‐adrenal axis and immune‐mediated inflammation. New England Journal of Medicine 1995;322(20):1351‐62. [PUBMED: 7715646] - PubMed
Cooper 2003
-
- Cooper MS, Stewart PM. Corticosteroid insufficiency in acutely ill patients. New England Journal of Medicine 2003;348(8):727‐34. [PUBMED: 12594318 ] - PubMed
Cronin 1995
-
- Cronin L, Cook DJ, Carlet J, Heyland DK, King D, Lansang MAD, et al. Corticosteroid treatment for sepsis: a critical appraisal and meta‐analysis of the literature. Critical Care Medicine 1995;23(8):1430‐9. [PUBMED: 7634816 ] - PubMed
de Kruif 2007
-
- Kruif MD, Lemaire LC, Giebelen IA, Zoelen MA, Pater JM, Pangaart PS, et al. Prednisolone dose‐dependently influences inflammation and coagulation during human endotoxemia. Journal of Immunology 2007;178(3):1845‐51. [PUBMED: 17237435] - PubMed
Devereaux 2001
-
- Devereaux PJ, Manns BJ, Ghali WA, Quan H, Lacchetti C, Montori VM, et al. Physician interpretations and textbook definitions of blinding terminology in randomized controlled trials. JAMA 2001;285(15):2000‐3. [PUBMED: 11308438 ] - PubMed
di Villa Bianca 2003
Duma 2004
-
- Duma D, Silva‐Santos JE, Assreuy J. Inhibition of glucocorticoid receptor binding by nitric oxide in endotoxemic rats. Critical Care Medicine 2004;32(11):2304–10. [PUBMED: 15640646] - PubMed
Egger 1997
Erschen 2007
-
- Ehrchen J, Steinmüller L, Barczyk K, Tenbrock K, Nacken W, Eisenacher M, et al. Glucocorticoids induce differentiation of a specifically activated, anti‐inflammatory subtype of human monocytes. Blood 2007;109(3):1265‐74. [PUBMED: 17018861] - PubMed
Fabian 1982
-
- Fabian TC, Patterson R. Steroid therapy in septic shock. Survival studies in a laboratory model. American Surgeon 1982;48(12):614‐7. [PUBMED: 6760756] - PubMed
Fang 2018
-
- Fang F, Zhang Y, Tang J, Lunsford LD, Li T, Tang R, et al. Association of corticosteroid treatment with outcomes in adult patients with sepsis: a systematic review and meta‐analysis. JAMA Internal Medicine 2019;179(2):213‐23. [DOI: 10.1001/jamainternmed.2018.5849; PUBMED: 30575845] - DOI - PMC - PubMed
Galigniana 1999
-
- Galigniana MD, Piwien‐Pilipuk G, Assreuy J. Inhibition of glucocorticoid receptor binding by nitric oxide. Molecular Pharmacology 1999;55(2):317–23. [PUBMED: 9927624] - PubMed
Goodwin 2013
GRADEpro GDT 2015 [Computer program]
-
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed 7 April 2019. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Guyatt 2008a
Guyatt 2008b
-
- Guyatt GH, Wyer P, Ioannidis J. When to believe a subgroup analysis. In: Guyatt G, Rennie R, Meade M, Cook D editor(s). User’s Guides to the Medical Literature: A Manual for Evidence‐Based Clinical Practice. New York: McGraw‐Hill, 2008.
Guyatt 2013
-
- Guyatt GH, Busse J. Methods commentary: risk of bias in randomized trials 1. Evidence Partners 2013 (accessed 15 December 2018):http://distillercer.com/resources/methodological‐resources/risk‐of‐biasc....
Heller 2003
-
- Heller AR, Heller SC, Borkenstein A, Stehr SN, Koch T. Modulation of host defence by hydrocortisone in stress doses during endotoxaemia. Intensive Care Medicine 2003;29(9):1456‐63. [PUBMED: 12879235 ] - PubMed
Heming 2018
Higgins 2011
-
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hotta 1986
Huang 1987
-
- Huang ZH, Gao H, Xu RB. Study on glucocorticoid receptors during intestinal ischemia shock and septic shock. Circulatory Shock 1987;23(1):27‐36. [PUBMED: 3690811] - PubMed
Ioannidis 2004
-
- Ioannidis JPA. Better reporting of harms in randomized trials: an extension of the CONSORT statement. Annals of Internal Medicine 2004;141(10):781‐8. [PUBMED: 15545678] - PubMed
Jaattela 1991
-
- Jaattela M, Ilvesmaki V, Voutilainen R, Stenman UH, Saksela E. Tumor necrosis factor as a potent inhibitor of adrenocorticotropin‐induced cortisol production and steroidogenic P450 enzyme gene expression in cultured human fetal adrenal cells. Endocrinology 1991;128(1):623‐9. [PUBMED: 1702707] - PubMed
Kadri 2017
Kalil 2011
-
- Kalil AC, Sun J. Low‐dose steroids for septic shock and severe sepsis: the use of Bayesian statistics to resolve clinical trial controversies. Intensive Care Medicine 2011;37(3):420–9. [PUBMED: 21243334] - PubMed
Kanczkowski 2013
Katsenos 2014
-
- Katsenos CS, Antonopoulou AN, Apostolidou EN, Ioakeimidou A, Kalpakou GT, Papanikolaou MN, et al. on behalf of the Hellenic Sepsis Study Group. Early administration of hydrocortisone replacement after the advent of septic shock: impact on survival and immune response. Critical Care Medicine 2014;42(7):1651‐7. [PUBMED: 24674923] - PubMed
Kellum 2007
-
- Kellum JA, Kong L, Fink MP, Weissfeld LA, Yealy DM, Pinsky MR, et al. Understanding the inflammatory cytokine response in pneumonia and sepsis: results of the Genetic and Inflammatory Markers of Sepsis (GenIMS) Study. Archives of Internal Medicine 2007;167(15):1655‐63. [PUBMED: 17698689] - PMC - PubMed
Lamontagne 2018
Lefering 1995
-
- Lefering R, Neugebauer EAM. Steroid controversy in sepsis and septic shock: a meta‐analysis. Critical Care Medicine 1995;23(7):1294‐303. [PUBMED: 7600840 ] - PubMed
Lipiner 2007
-
- Lipiner D, Sprung CL, Laterre PF, Weiss Y, Goodman SV, Vogeser M, et al. Adrenal function in sepsis: the retrospective CORTICUS cohort study. Critical Care Medicine 2007;35(4):1012‐8. [PUBMED: 17334243] - PubMed
Lowenberg 2005
-
- Lowenberg M, Tuynman J, Bilderbeek J, Gaber T, Buttgereit F, Deventer S, et al. Rapid immunosuppressive effects of glucocorticoids mediated through Lck and Fyn. Blood 2005;106(5):1703‐10. [PUBMED: 15899916] - PubMed
Marik 2008
-
- Marik PE, Pastores SM, Annane D, Meduri GU, Sprung CL, Arlt W, et al. Recommendations for the diagnosis and management of corticosteroid insufficiency in critically ill adult patients: consensus statements from an international task force by the American College of Critical Care Medicine. Critical Care Medicine 2008;36(6):1937–49. [PUBMED: 18496365] - PubMed
Meduri 1998a
-
- Meduri GU, Chrousos GP. Duration of glucocorticoid treatment and outcome in sepsis: is the right drug used the wrong way?. Chest 1998;114(2):355‐60. [PUBMED: 9726712] - PubMed
Melby 1958
Menon 2013
-
- Menon K, McNally D, Choong K, Sampson M. A systematic review and meta‐analysis on the effect of steroids in pediatric shock. Pediatric Critical Care Medicine 2013;14(5):474‐80. [PUBMED: 23867428] - PubMed
Molijn 1995
-
- Molijn GJ, Koper JW, Uffelen CJ, Jong FH, Brinkmann AO, Bruining HA, et al. Temperature‐induced down‐regulation of the glucocorticoid receptor in peripheral blood mononuclear leucocyte in patients with sepsis or septic shock. Clinical Endocrinology 1995;43(2):197‐203. [PUBMED: 7554315 ] - PubMed
Moran 2010
Ni 2018
-
- Ni YN, Liu YM, Wang YW, Liang BM, Liang ZA. Can corticosteroids reduce the mortality of patients with severe sepsis? A systematic review and meta‐analysis. American Journal of Emergency Medicine 2018;S0735‐6757:30953‐7. [PUBMED: 30522935] - PubMed
Nishida 2018
Norman 2004
-
- Norman AW, Mizwicki MT, Norman DP. Steroid‐hormone rapid actions, membrane receptors, and a conformational ensemble model. Nature Reviews. Drug Discovery 2004;3(1):27‐41. [PUBMED: 14708019] - PubMed
Park 2012
Parrillo 1993
-
- Parrillo JE. Pathogenic mechanisms of septic shock. New England Journal of Medicine 1993;328(20):1471‐7. [PUBMED: 8479467] - PubMed
Peters 2008
-
- Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Contour‐enhanced meta‐analysis funnel plots help distinguish publication bias from other causes of asymmetry. Journal of Clinical Epidemiology 2008;61(10):991‐6. [PUBMED: 18538991] - PubMed
Polito 2011
Review Manager 2014 [Computer program]
-
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rhee 2017
Rhen 2005
-
- Rhen T, Cidlowski JA. Antiinflammatory action of glucocorticoids: new mechanisms for old drugs. New England Journal of Medicine 2005;353(16):1711‐23. [PUBMED: 16236742] - PubMed
Rhodes 2017
-
- Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, et al. Surviving Sepsis Campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Medicine 2017;43(3):304‐77. [PUBMED: 28101605] - PubMed
Rochwerg 2018
-
- Rochwerg B, Oczkowski SJ, Siemieniuk RAC, Agoritsas T, Belley‐Cote E, D'Aragon F, et al. Corticosteroids in sepsis: an updated systematic review and meta‐analysis. Critical Care Medicine 2018;46(9):1411‐20. [PUBMED: 29979221] - PubMed
Rothwell 1991
-
- Rothwell PM, Udwadia ZF, Lawler PG. Cortisol response to corticotropin and survival in septic shock. Lancet 1991;337(8741):582‐3. [PUBMED: 1671944] - PubMed
Rygard 2018
-
- Rygård SL, Butler E, Granholm A, Møller MH, Cohen J, Finfer S, et al. Low‐dose corticosteroids for adult patients with septic shock: a systematic review with meta‐analysis and trial sequential analysis. Intensive Care Medicine 2018;44(7):1003‐16. [PUBMED: 29761216] - PubMed
Sharshar 2003
-
- Sharshar T, Gray F, Lorin de la Grandmaison G, Hopkinson NS, Ross E, Dorandeu A, et al. Apoptosis of neurons in cardiovascular autonomic centres triggered by inducible nitric oxide synthase after death from septic shock. Lancet 2003;362(9398):1799‐805. [PUBMED: 14654318] - PubMed
Sherwin 2012
-
- Sherwin RL, Garcia AJ, Bilkovski R. Do low‐dose corticosteroids improve mortality or shock reversal in patients with septic shock? A systematic review and position statement prepared for the American Academy of Emergency Medicine. Journal of Emergency Medicine 2012;43(1):7‐12. [PUBMED: 22221983] - PubMed
Singer 2016
Stata 2015 [Computer program]
-
- StataCorp. 2015. Stata Statistical Software: Release 14. College Station, TX: StataCorp LP, 2015.
Tavaré 2017
Tsao 2004
-
- Tsao CM, Ho ST, Chen A, Wang JJ, Li CY, Tsai SK, et al. Low‐dose dexamethasone ameliorates circulatory failure and renal dysfunction in conscious rats with endotoxemia. Shock 2004;21(5):484‐91. [PUBMED: 15087827] - PubMed
Vachharajani 2006
-
- Vachharajani V, Vital S, Russell J, Scott LK, Granger DN. Glucocorticoids inhibit the cerebral microvascular dysfunction associated with sepsis in obese mice. Microcirculation 2006;13(6):477‐87. [PUBMED: 16864414 ] - PubMed
van der Poll 2017
-
- Poll T, Veerdonk FL, Scicluna BP, Netea MG. The immunopathology of sepsis and potential therapeutic targets. Nature Reviews. Immunology 2017;17(7):407‐20. [PUBMED: 28436424] - PubMed
Varga 2008
-
- Varga G, Ehrchen J, Tsianakas A, Tenbrock K, Rattenholl A, Seeliger S, et al. Glucocorticoids induce an activated, anti‐inflammatory monocyte subset in mice that resembles myeloid‐derived suppressor cells. Journal of Leukocyte Biology 2008;84(3):644‐50. [PUBMED: 18611985] - PubMed
Vincent 1996
-
- Vincent JL, Moreno R, Takala J, Willatts S, Mendonça A, Bruining H, et al. The SOFA (Sepsis‐related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis‐Related Problems of the European Society of Intensive Care Medicine. Intensive Care Medicine 1996;22(7):707‐10. [PUBMED: 8844239] - PubMed
Vincent 2013
WHO 2018
-
- WHO. Improving prevention, diagnosis and clinical management of sepsis. https://www.who.int/servicedeliverysafety/areas/sepsis/en/#.XAAf362Zqkk..... 2018. Accessed 29 November 2018.
References to other published versions of this review
Annane 2004
Annane 2009
-
- Annane D, Bellissant E, Bollaert PE, Briegel J, Confalonieri M, Gaudio R, et al. Corticosteroids in the treatment of severe sepsis and septic shock in adults: a systematic review. JAMA 2009;301:2362‐75. [PUBMED: 19509383 ] - PubMed
Annane 2015
Gibbison 2017
Rochwerg 2018a
-
- Rochwerg B, Oczkowski SJ, Siemieniuk RAC, Agoritsas T, Belley‐Cote E, D'Aragon F, et al. Corticosteroids in sepsis: an updated systematic review and meta‐analysis. Critical Care Medicine 2018;46:1411‐20. [PUBMED: 29979221] - PubMed

