Estimation of an Appropriate Dose of Trazodone for Pediatric Insomnia and the Potential for a Trazodone-Atomoxetine Interaction
- PMID: 31808613
- PMCID: PMC7020267
- DOI: 10.1002/psp4.12480
Estimation of an Appropriate Dose of Trazodone for Pediatric Insomnia and the Potential for a Trazodone-Atomoxetine Interaction
Abstract
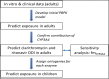
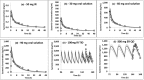
There is a paucity of clinical trials for the treatment of pediatric insomnia. This study was designed to predict the doses of trazodone to guide dosing in a clinical trial for pediatric insomnia using physiologically-based pharmacokinetic (PBPK) modeling. Data on the pharmacokinetics of trazodone in children are currently lacking. The interaction potential between trazodone and atomoxetine was also predicted. Doses predicted in the following age groups, with exposures corresponding to adult dosages of 30, 75, and 150 mg once a day (q.d.), respectively, were: (i) 2- to 6-year-old group, doses of 0.35, 0.8, and 1.6 mg/kg q.d.; (ii) >6- to 12-year-old group, doses of 0.4, 1.0, and 1.9 mg/kg q.d.; (iii) >12- to 17-year-old group, doses of 0.4, 1.1, and 2.1 mg/kg q.d. An interaction between trazodone and atomoxetine was predicted to be unlikely. Clinical trials based on the aforementioned predicted dosing are currently in progress, and pharmacokinetic data obtained will enable further refinement of the PBPK models.
© 2019 ANGELINI S.p.A. CPT: Pharmacometrics & Systems Pharmacology published by Wiley Periodicals, Inc. on behalf of the American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
L.O., R.P., V.P., F.C., F.G. and S.T. are employees of Angelini S.p.A. A.B.K. and M.C. are employees of Certara UK, Simcyp Division.
Figures

Similar articles
-
The efficacy and tolerability of once-daily controlled-release trazodone for depressed mood, anxiety, insomnia, and suicidality in major depressive disorder.Psychopharmacol Bull. 2009;42(4):5-22. Psychopharmacol Bull. 2009. PMID: 20581790 Clinical Trial.
-
Effect of trazodone in a single dose before bedtime for sleep disorders accompanied by a depressive state: dose-finding study with no concomitant use of hypnotic agent.Psychiatry Clin Neurosci. 1999 Apr;53(2):193-4. doi: 10.1046/j.1440-1819.1999.00532.x. Psychiatry Clin Neurosci. 1999. PMID: 10459686 Clinical Trial.
-
Safety, tolerability, and pharmacokinetics of once-daily trazodone extended-release caplets in healthy subjects.Int J Clin Pharmacol Ther. 2011 Dec;49(12):730-43. doi: 10.5414/cp201546. Int J Clin Pharmacol Ther. 2011. PMID: 22122815 Clinical Trial.
-
[Personalized treatment of depression phenotypes: role of trazodone in depression with insomnia].Riv Psichiatr. 2020 Nov-Dec;55(6):371-379. doi: 10.1708/3503.34896. Riv Psichiatr. 2020. PMID: 33349731 Review. Italian.
-
[Trazodone Contramid® in clinical practice: personalizing antidepressant intervention].Riv Psichiatr. 2016 Jul-Aug;51(4):123-128. doi: 10.1708/2342.25112. Riv Psichiatr. 2016. PMID: 27727261 Review. Italian.
Cited by
-
Physiologically-based pharmacokinetic modeling to predict drug interactions of lemborexant with CYP3A inhibitors.CPT Pharmacometrics Syst Pharmacol. 2021 May;10(5):455-466. doi: 10.1002/psp4.12606. Epub 2021 May 1. CPT Pharmacometrics Syst Pharmacol. 2021. PMID: 33704920 Free PMC article.
-
Physiologically Based Pharmacokinetic Models Are Effective Support for Pediatric Drug Development.AAPS PharmSciTech. 2021 Jul 26;22(6):208. doi: 10.1208/s12249-021-02076-w. AAPS PharmSciTech. 2021. PMID: 34312742 Free PMC article. Review.
-
Estimation of brain receptor occupancy for trazodone immediate release and once a day formulations.Clin Transl Sci. 2022 Jun;15(6):1417-1429. doi: 10.1111/cts.13253. Epub 2022 Mar 2. Clin Transl Sci. 2022. PMID: 35233913 Free PMC article.
-
Pharmacokinetic and pharmacodynamic principles: unique considerations for optimal design of neonatal clinical trials.Front Pediatr. 2024 Jan 12;11:1345969. doi: 10.3389/fped.2023.1345969. eCollection 2023. Front Pediatr. 2024. PMID: 38283405 Free PMC article. Review.
-
Physiologically Based Pharmacokinetic Modeling to Predict Drug-Drug Interactions of Soticlestat as a Victim of CYP Induction and Inhibition, and as a Perpetrator of CYP and P-Glycoprotein Inhibition.Clin Pharmacol Drug Dev. 2025 May;14(5):368-381. doi: 10.1002/cpdd.1526. Epub 2025 Mar 27. Clin Pharmacol Drug Dev. 2025. PMID: 40145722 Free PMC article.
References
-
- Angriman, M. , Caravale, B. , Novelli, L. , Ferri, R. & Bruni, O. Sleep in children with neurodevelopmental disabilities. Neuropediatrics 46, 199–210 (2015). - PubMed
-
- Hvolby, A. , Jørgensen, J. & Bilenberg, N. Actigraphic and parental reports of sleep difficulties in children with attention‐deficit/hyperactivity disorder. Arch. Pediatr. Adolesc. Med. 162, 323–329 (2008). - PubMed
-
- Miano, S. & Ferri, R. Epidemiology and management of insomnia in children with autistic spectrum disorders. Paediatr. Drugs 12, 75–84 (2010). - PubMed
-
- Blackmer, A.B. & Feinstein, J.A. Management of sleep disorders in children with neurodevelopmental disorders: a review. Pharmacotherapy 36, 84–98 (2016). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

