MRD evaluation of AML in clinical practice: are we there yet?
- PMID: 31808906
- PMCID: PMC6913462
- DOI: 10.1182/hematology.2019000060
MRD evaluation of AML in clinical practice: are we there yet?
Abstract
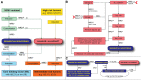
MRD technologies increase our ability to measure response in acute myeloid leukemia (AML) beyond the limitations of morphology. When applied in clinical trials, molecular and immunophenotypic MRD assays have improved prognostic precision, providing a strong rationale for their use to guide treatment, as well as to measure its effectiveness. Initiatives such as those from the European Leukemia Network now provide a collaborative knowledge-based framework for selection and implementation of MRD assays most appropriate for defined genetic subgroups. For patients with mutated-NPM1 AML, quantitative polymerase chain reaction (qPCR) monitoring of mutated-NPM1 transcripts postinduction and sequentially after treatment has emerged as a highly sensitive and specific tool to predict relapse and potential benefit from allogeneic transplant. Flow cytometric MRD after induction is prognostic across genetic risk groups and can identify those patients in the wild-type NPM1 intermediate AML subgroup with a very high risk for relapse. In parallel with these data, advances in genetic profiling have extended understanding of the etiology and the complex dynamic clonal nature of AML, as well as created the opportunity for MRD monitoring using next-generation sequencing (NGS). NGS AML MRD detection can stratify outcomes and has potential utility in the peri-allogeneic transplant setting. However, there remain challenges inherent in the NGS approach of multiplex quantification of mutations to track AML MRD. Although further development of this methodology, together with orthogonal testing, will clarify its relevance for routine clinical use, particularly for patients lacking a qPCR genetic target, established validated MRD assays can already provide information to direct clinical practice.
© 2019 by The American Society of Hematology. All rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: C.S.H. has received laboratory research funding from Merck and Sellas. S.D.F. declares no competing financial interests.
Figures


Similar articles
-
Advantages of a genomic DNA-based next-generation sequencing assay for detection of mutant NPM1 measurable residual disease in AML.Blood Adv. 2025 Mar 11;9(5):1069-1077. doi: 10.1182/bloodadvances.2024014490. Blood Adv. 2025. PMID: 39637308 Free PMC article.
-
Deep NPM1 Sequencing Following Allogeneic Hematopoietic Cell Transplantation Improves Risk Assessment in Adults with NPM1-Mutated AML.Biol Blood Marrow Transplant. 2018 Aug;24(8):1615-1620. doi: 10.1016/j.bbmt.2018.04.017. Epub 2018 Apr 21. Biol Blood Marrow Transplant. 2018. PMID: 29684564
-
Minimal/Measurable Residual Disease Monitoring in NPM1-Mutated Acute Myeloid Leukemia: A Clinical Viewpoint and Perspectives.Int J Mol Sci. 2018 Nov 6;19(11):3492. doi: 10.3390/ijms19113492. Int J Mol Sci. 2018. PMID: 30404199 Free PMC article. Review.
-
Flow cytometric immunophenotypic alterations of persistent clonal haematopoiesis in remission bone marrows of patients with NPM1-mutated acute myeloid leukaemia.Br J Haematol. 2021 Mar;192(6):1054-1063. doi: 10.1111/bjh.17347. Epub 2021 Feb 22. Br J Haematol. 2021. PMID: 33618432 Free PMC article. Clinical Trial.
-
Minimal residual disease in acute myelogenous leukemia.Int J Lab Hematol. 2017 May;39 Suppl 1(Suppl 1):53-60. doi: 10.1111/ijlh.12670. Int J Lab Hematol. 2017. PMID: 28447422 Free PMC article. Review.
Cited by
-
Quantification of measurable residual disease using duplex sequencing in adults with acute myeloid leukemia.Haematologica. 2024 Feb 1;109(2):401-410. doi: 10.3324/haematol.2023.283520. Haematologica. 2024. PMID: 37534515 Free PMC article.
-
Accumulation of STR-Loci Aberrations in Subclones of Jurkat Cell Line as a Model of Tumor Clonal Evolution.Genes (Basel). 2023 Feb 24;14(3):571. doi: 10.3390/genes14030571. Genes (Basel). 2023. PMID: 36980843 Free PMC article.
-
Acute Myeloid Leukemia-Use of Measurable Residual Disease to Improve Outcomes.Adv Exp Med Biol. 2025;1475:129-148. doi: 10.1007/978-3-031-84988-6_7. Adv Exp Med Biol. 2025. PMID: 40488827 Review.
-
Measurable residual mutated IDH1 before allogeneic transplant for acute myeloid leukemia.Bone Marrow Transplant. 2025 Feb;60(2):154-160. doi: 10.1038/s41409-024-02447-4. Epub 2024 Nov 6. Bone Marrow Transplant. 2025. PMID: 39506075 Free PMC article.
-
Risk-directed therapy based on genetics and MRD improves the outcomes of AML1-ETO-positive AML patients, a multi-center prospective cohort study.Blood Cancer J. 2023 Nov 13;13(1):168. doi: 10.1038/s41408-023-00941-4. Blood Cancer J. 2023. PMID: 37957175 Free PMC article. No abstract available.
References
-
- Hourigan CS, Gale RP, Gormley NJ, Ossenkoppele GJ, Walter RB. Measurable residual disease testing in acute myeloid leukaemia. Leukemia. 2017;31(7):1482-1490. - PubMed
-
- Ivey A, Hills RK, Simpson MA, et al. ; UK National Cancer Research Institute AML Working Group. Assessment of minimal residual disease in standard-risk AML. N Engl J Med. 2016;374(5):422-433. - PubMed
-
- Balsat M, Renneville A, Thomas X, et al. . Postinduction minimal residual disease predicts outcome and benefit from allogeneic stem cell transplantation in acute myeloid leukemia with NPM1 mutation: a study by the Acute Leukemia French Association Group. J Clin Oncol. 2017;35(2):185-193. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

