Association of Patient, Prescriber, and Region With the Initiation of First Prescription of Biologic Disease-Modifying Antirheumatic Drug Among Older Patients With Rheumatoid Arthritis and Identical Health Insurance Coverage
- PMID: 31808927
- PMCID: PMC6902765
- DOI: 10.1001/jamanetworkopen.2019.17053
Association of Patient, Prescriber, and Region With the Initiation of First Prescription of Biologic Disease-Modifying Antirheumatic Drug Among Older Patients With Rheumatoid Arthritis and Identical Health Insurance Coverage
Abstract
Importance: Prescribing the first biologic treatment for rheumatoid arthritis (RA) is an important decision for patients, their physicians, and payers, with considerable costs and clinical implications. Conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) have known effectiveness and safety profiles and are less expensive; therefore, determining the variables contributing to csDMARD treatment duration is an essential question for patients, physicians, and payers.
Objectives: To describe access to the first biologic DMARD prescription in a population of patients with RA and identical comprehensive health insurance coverage in Ontario, Canada, and to explore the associations of patient, prescriber, and geographic region with differences in time to first biologic prescription.
Design, setting, and participants: This cohort study of incident patients with RA used administrative data with surveillance and patient-level data collected at yearly intervals. A total of 17 672 patients were included in the study; they were residents of Ontario, Canada, had an incident RA diagnosis at age 67 or older between 2002 and 2015, and received at least 1 csDMARD. Data were analyzed in November 2017.
Exposure: Patient variables were age, sex, disease duration, socioeconomic status, distance to care, and supply of care in the patient's area of residence. Prescriber covariates were year of graduation, specialty of practice, and supply of rheumatologic care in the patient's geographic region.
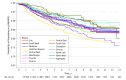
Main outcomes and measures: Time from first csDMARD prescription to receipt of first biologic medication.
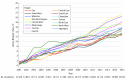
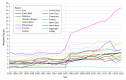
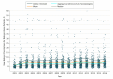
Results: Of 17 672 patients, 11 598 (65.6%) were women, and the mean (SD) age was 75.2 (5.8) years. Characteristics associated with longer time to receipt of a biologic prescription were older age (HR for every 5-year increase, 0.66; 95% CI, 0.62-0.71; P < .001), male sex (HR, 0.76; 95% CI, 0.66-0.89; P < .001), and distance to the nearest rheumatologist (HR per 10-km increase, 0.99; 95% CI, 0.98-0.99; P < .001). Prescribers were primarily rheumatologists (151 of 214 [70.6%]) and primary care physicians (26 of 214 [12.1%]). After adjusting for the number of patients eligible to receive biologic DMARDs, rheumatologists' preferences (ie, yearly prescription rates) for using biologic DMARDs increased over time, from 1.7% in 2001 to 4.9% in 2015. After adjusting for calendar year and patient-, prescriber-, and region-level characteristics, substantial variation between prescribers in rates of prescribing a first biologic DMARD were found (65% variance).
Conclusions and relevance: This study found variation in time to receipt of first biologic DMARD after prescription of first csDMARD in a population with RA after adjustment for individual-level patient, prescriber, and geographic area covariates, despite identical universal health insurance coverage.
Conflict of interest statement
Figures
Comment in
-
Which Patients With Rheumatoid Arthritis Will Start Biologics, How Soon, and Why-Much to Learn From a Universal Coverage Setting.JAMA Netw Open. 2019 Dec 2;2(12):e1917065. doi: 10.1001/jamanetworkopen.2019.17065. JAMA Netw Open. 2019. PMID: 31808919 No abstract available.
Similar articles
-
Patterns of the initiation of disease-modifying antirheumatic drugs in incident rheumatoid arthritis: a German perspective based on nationwide ambulatory drug prescription data.Rheumatol Int. 2018 Nov;38(11):2111-2120. doi: 10.1007/s00296-018-4161-7. Epub 2018 Oct 10. Rheumatol Int. 2018. PMID: 30306254 Free PMC article.
-
Impact of residential area on the management of rheumatoid arthritis patients initiating their first biologic DMARD: Results from the Ontario Best Practices Research Initiative (OBRI).Medicine (Baltimore). 2019 May;98(20):e15517. doi: 10.1097/MD.0000000000015517. Medicine (Baltimore). 2019. PMID: 31096451 Free PMC article.
-
Medicare beneficiary panel characteristics associated with high Part D biologic disease-modifying anti-rheumatic drug prescribing for older adults among rheumatologists.Medicine (Baltimore). 2021 Apr 23;100(16):e25644. doi: 10.1097/MD.0000000000025644. Medicine (Baltimore). 2021. PMID: 33879745 Free PMC article.
-
Conventional Disease-Modifying Antirheumatic Drugs for the Treatment of Rheumatoid Arthritis [Internet].Ottawa (ON): Canadian Agency for Drugs and Technologies in Health; 2021 May. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health; 2021 May. PMID: 36130027 Free Books & Documents. Review.
-
Singapore Chapter of Rheumatologists Consensus Statement on the Eligibility for Government Subsidy of Biologic Disease Modifying Antirheumatic Agents for Treatment of Rheumatoid Arthritis (RA).Ann Acad Med Singap. 2014 Aug;43(8):400-11. Ann Acad Med Singap. 2014. PMID: 25244989 Review.
Cited by
-
Elicitation of Rheumatologist Preferences for the Treatment of Patients with Rheumatoid Arthritis After the Failure of a First Conventional Synthetic Disease-Modifying Anti-Rheumatic Agent.Rheumatol Ther. 2021 Jun;8(2):921-935. doi: 10.1007/s40744-021-00311-1. Epub 2021 May 3. Rheumatol Ther. 2021. PMID: 33939171 Free PMC article.
-
Comanagement with rheumatology and prescription biologics filled during pregnancy in women with rheumatic diseases: a retrospective analysis of US administrative claims data.BMJ Open. 2022 Dec 22;12(12):e065189. doi: 10.1136/bmjopen-2022-065189. BMJ Open. 2022. PMID: 36549721 Free PMC article.
-
Development of a Machine Learning Model to Predict the Use of Surgery in Patients With Rheumatoid Arthritis.Arthritis Care Res (Hoboken). 2024 May;76(5):636-643. doi: 10.1002/acr.25287. Epub 2024 Mar 19. Arthritis Care Res (Hoboken). 2024. PMID: 38155538 Free PMC article.
-
Phenolic-Compound-Rich Opuntia littoralis Ethyl Acetate Extract Relaxes Arthritic Symptoms in Collagen-Induced Mice Model via Bone Morphogenic Markers.Nutrients. 2022 Dec 17;14(24):5366. doi: 10.3390/nu14245366. Nutrients. 2022. PMID: 36558525 Free PMC article.
-
Temporal and regional variation in the use of biologic and targeted synthetic DMARDs for rheumatoid arthritis: a nationwide cohort study.Rheumatology (Oxford). 2025 May 1;64(5):2432-2441. doi: 10.1093/rheumatology/keae607. Rheumatology (Oxford). 2025. PMID: 39485485 Free PMC article.
References
-
- Bykerk VP, Akhavan P, Hazlewood GS, et al. ; Canadian Rheumatology Association . Canadian Rheumatology Association recommendations for pharmacological management of rheumatoid arthritis with traditional and biologic disease-modifying antirheumatic drugs. J Rheumatol. 2012;39(8):1559-1582. doi:10.3899/jrheum.110207 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

