Pathophysiology and targets for treatment in hereditary galactosemia: A systematic review of animal and cellular models
- PMID: 31808946
- PMCID: PMC7317974
- DOI: 10.1002/jimd.12202
Pathophysiology and targets for treatment in hereditary galactosemia: A systematic review of animal and cellular models
Abstract
Since the first description of galactosemia in 1908 and despite decades of research, the pathophysiology is complex and not yet fully elucidated. Galactosemia is an inborn error of carbohydrate metabolism caused by deficient activity of any of the galactose metabolising enzymes. The current standard of care, a galactose-restricted diet, fails to prevent long-term complications. Studies in cellular and animal models in the past decades have led to an enormous progress and advancement of knowledge. Summarising current evidence in the pathophysiology underlying hereditary galactosemia may contribute to the identification of treatment targets for alternative therapies that may successfully prevent long-term complications. A systematic review of cellular and animal studies reporting on disease complications (clinical signs and/or biochemical findings) and/or treatment targets in hereditary galactosemia was performed. PubMed/MEDLINE, EMBASE, and Web of Science were searched, 46 original articles were included. Results revealed that Gal-1-P is not the sole pathophysiological agent responsible for the phenotype observed in galactosemia. Other currently described contributing factors include accumulation of galactose metabolites, uridine diphosphate (UDP)-hexose alterations and subsequent impaired glycosylation, endoplasmic reticulum (ER) stress, altered signalling pathways, and oxidative stress. galactokinase (GALK) inhibitors, UDP-glucose pyrophosphorylase (UGP) up-regulation, uridine supplementation, ER stress reducers, antioxidants and pharmacological chaperones have been studied, showing rescue of biochemical and/or clinical symptoms in galactosemia. Promising co-adjuvant therapies include antioxidant therapy and UGP up-regulation. This systematic review provides an overview of the scattered information resulting from animal and cellular studies performed in the past decades, summarising the complex pathophysiological mechanisms underlying hereditary galactosemia and providing insights on potential treatment targets.
Keywords: animal models; cellular models; hereditary galactosemia; pathophysiology; treatment targets.
© 2019 The Authors. Journal of Inherited Metabolic Disease published by John Wiley & Sons Ltd on behalf of SSIEM.
Conflict of interest statement
The authors declare no potential conflict of interest.
Figures
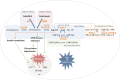
 , indicating a treatment approach that has been evaluated in the cellular and animal models.
, indicating a treatment approach that has been evaluated in the cellular and animal models.  , inhibition.
, inhibition.  , induction. GALK deficiency was studied in yeast (Y), fruitfly (F) and mouse (M). GALT deficiency was studied in patient cell cultures (C), bacteria (B), yeast, fruitfly, zebrafish (ZF) and mouse. GALE deficiency was studied in bacteria, yeast, nematode (N) and fruitfly. AR, aldose reductase, ER, endoplasmic reticulum, Gal‐1‐P, galactose‐1‐phosphate, GALE, UDP‐galactose 4′‐epimerase, GALK, galactokinase, GALT, galactose‐1‐phosphate uridylyltransferase, GALM, galactose mutarotase, GDH, galactose dehydrogenase, Glc‐1‐P, glucose‐1‐phosphate, PI3/Akt, phosphatidylinositol‐4,5‐bisphosphate 3‐kinase/protein kinase B, PPi, inorganic pyrophosphate, ROS, reactive oxygen species, RP, ribosomal protein, UDP‐Gal, uridine diphosphate‐galactose, UDP‐GalNAc, UDP‐N‐acetylgalactosamine, UDP‐Glc, uridine diphosphate‐glucose, UDP‐GlcNAc, UDP‐N‐acetylglucosamine, UGP, UDP‐glucose pyrophosphorylase, UPR, unfolded protein response, UTP, uridine‐5′‐triphosphate
, induction. GALK deficiency was studied in yeast (Y), fruitfly (F) and mouse (M). GALT deficiency was studied in patient cell cultures (C), bacteria (B), yeast, fruitfly, zebrafish (ZF) and mouse. GALE deficiency was studied in bacteria, yeast, nematode (N) and fruitfly. AR, aldose reductase, ER, endoplasmic reticulum, Gal‐1‐P, galactose‐1‐phosphate, GALE, UDP‐galactose 4′‐epimerase, GALK, galactokinase, GALT, galactose‐1‐phosphate uridylyltransferase, GALM, galactose mutarotase, GDH, galactose dehydrogenase, Glc‐1‐P, glucose‐1‐phosphate, PI3/Akt, phosphatidylinositol‐4,5‐bisphosphate 3‐kinase/protein kinase B, PPi, inorganic pyrophosphate, ROS, reactive oxygen species, RP, ribosomal protein, UDP‐Gal, uridine diphosphate‐galactose, UDP‐GalNAc, UDP‐N‐acetylgalactosamine, UDP‐Glc, uridine diphosphate‐glucose, UDP‐GlcNAc, UDP‐N‐acetylglucosamine, UGP, UDP‐glucose pyrophosphorylase, UPR, unfolded protein response, UTP, uridine‐5′‐triphosphate
References
-
- Holden HM, Rayment I, Thoden JB. Structure and function of enzymes of the Leloir pathway for galactose metabolism. J Biol Chem. 2003;278:43885‐43888. - PubMed
-
- Charlwood J, Clayton P, Keir G, Mian N, Winchester B. Defective galactosylation of serum transferrin in galactosemia. Glycobiology. 1998;8(4):351‐357. - PubMed
-
- Coss KP, Treacy EP, Cotter EJ, et al. Systemic gene dysregulation in classical Galactosaemia: is there a central mechanism? Mol Genet Metab. 2014;113(3):177‐187. - PubMed
-
- Lai K, Langley SD, Khwaja FW, Schmitt EW, Elsas LJ. GALT deficiency causes UDP‐hexose deficit in human galactosemic cells. Glycobiology. 2003;13(4):285‐294. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

