Self-Amplified Photodynamic Therapy through the 1 O2 -Mediated Internalization of Photosensitizers from a Ppa-Bearing Block Copolymer
- PMID: 31808983
- PMCID: PMC7028480
- DOI: 10.1002/anie.201914434
Self-Amplified Photodynamic Therapy through the 1 O2 -Mediated Internalization of Photosensitizers from a Ppa-Bearing Block Copolymer
Abstract
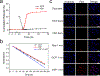
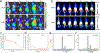
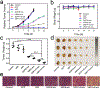
Nanocarriers are employed to deliver photosensitizers for photodynamic therapy (PDT) through the enhanced penetration and retention effect, but disadvantages including the premature leakage and non-selective release of photosensitizers still exist. Herein, we report a 1 O2 -responsive block copolymer (POEGMA-b-P(MAA-co-VSPpaMA) to enhance PDT via the controllable release of photosensitizers. Once nanoparticles formed by the block copolymer have accumulated in a tumor and have been taken up by cancer cells, pyropheophorbide a (Ppa) could be controllably released by singlet oxygen (1 O2 ) generated by light irradiation, enhancing the photosensitization. This was demonstrated by confocal laser scanning microscopy and in vivo fluorescence imaging. The 1 O2 -responsiveness of POEGMA-b-P(MAA-co-VSPpaMA) block copolymer enabled the realization of self-amplified photodynamic therapy by the regulation of Ppa release using NIR illumination. This may provide a new insight into the design of precise PDT.
Keywords: block copolymer; drug delivery; photodynamic therapy; reactive oxygen species; self-amplification.
© 2019 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures



References
-
- Dolmans DE, Fukumura D, Jain RK, Nat. Rev. Cancer 2003, 3, 380–387. - PubMed
-
- Lucky SS, Soo KC, Zhang Y, Chem. Rev 2015, 115, 1990–2042. - PubMed
-
- Han Y, Chen Z, Zhao H, Zha Z, Ke W, Wang Y, Ge Z, J. Controlled Release 2018, 284, 15–25; - PubMed
- Zheng L, Zhang X, Wang Y, Liu F, Peng J, Zhao X, Yang H, Ma L, Wang B, Chang C, Wei H, Biomacromolecules 2018, 19, 3874–3882; - PubMed
- Seah GL, Yu JH, Yang MY, Kim WJ, Kim JH, Park K, Cho JW, Kim JS, Nam YS, J. Controlled Release 2018, 286, 240–253; - PubMed
- Saravanakumar G, Lee J, Kim J, Kim WJ, Chem. Commun 2015, 51, 9995–9998. - PubMed
-
- Jin CS, Lovell JF, Chen J, Zheng G, ACS Nano 2013, 7, 2541–2550; - PMC - PubMed
- Yu B, Goel S, Ni D, Ellison PA, Siamof CM, Jiang D, Cheng L, Kang L, Yu F, Liu Z, Barnhart TE, He Q, Zhang H, Cai W, Adv. Mater 2018, 30, 1704934; - PMC - PubMed
- Jia HR, Zhu YX, Xu KF, Liu X, Wu FG, J. Controlled Release 2018, 286, 103–113; - PubMed
- Rahman MM, Ueda M, Hirose T, Ito Y, J. Am. Chem. Soc 2018, 140, 17956–17961; - PubMed
- Zhang K, Zhang Y, Meng X, Lu H, Chang H, Dong H, Zhang X, Biomaterials 2018, 185, 301–309; - PubMed
- Lovell JF, Jin CS, Huynh E, Jin H, Kim C, Rubinstein JL, Chan WC, Cao W, Wang LV, Zheng G, Nat. Mater 2011, 10, 324–332. - PubMed
-
- Barnard A, Posocco P, Pricl S, Calderon M, Haag R, Hwang ME, Shum VW, Pack DW, Smith DK, J. Am. Chem. Soc 2011, 133, 20288–20300; - PubMed
- Lin TY, Li Y, Liu Q, Chen JL, Zhang H, Lac D, Zhang H, Ferrara KW, Wachsmann-Hogiu S, Li T, Airhart S, deVere White R, Lam KS, Pan CX, Biomaterials 2016, 104, 339–351; - PMC - PubMed
- Zhou Y, Huang W, Liu J, Zhu X, Yan D, Adv. Mater 2010, 22, 4567–4590; - PubMed
- Li Y, Lin TY, Luo Y, Liu Q, Xiao W, Guo W, Lac D, Zhang H, Feng C, Wachsmann-Hogiu S, Walton JH, Cherry SR, Rowland DJ, Kukis D, Pan C, Lam KS, Nat. Commun 2014, 5, 4712. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

