Programmable Assembly of Adeno-Associated Virus-Antibody Composites for Receptor-Mediated Gene Delivery
- PMID: 31809024
- PMCID: PMC7676631
- DOI: 10.1021/acs.bioconjchem.9b00790
Programmable Assembly of Adeno-Associated Virus-Antibody Composites for Receptor-Mediated Gene Delivery
Abstract
Adeno-associated virus (AAV) has emerged as a viral gene delivery vector that is safe in humans, able to infect both dividing and arrested cells and drive long-term expression (>6 months). Unfortunately, the naturally evolved properties of many AAV serotypes-including low cell type specificity and largely overlapping tropism-are mismatched to applications that require cell type-specific infection, such as neural circuit mapping or precision gene therapy. A variety of approaches to redirect AAV tropism exist, but there is still the need for a universal solution for directing AAV tropism toward user-defined cellular receptors that does not require extensive case-by-case optimization and works with readily available components. Here, we report AAV engineering approaches that enable programmable receptor-mediated gene delivery. First, we genetically encode small targeting scaffolds into a variable region of an AAV capsid and show that this redirects tropism toward the receptor recognized by these targeting scaffolds and also renders this AAV variant resistant to neutralizing antibodies present in nonhuman primate serum. We then simplify retargeting of tropism by engineering the same variable loop to encode a HUH tag, which forms a covalent bond to single-stranded DNA oligos conjugated to store-bought antibodies. We demonstrate that retargeting this HUH-AAVs toward different receptors is as simple as "arming" a premade noninfective AAV template with a different antibody in a conjugation process that uses widely available reagents and requires no optimization or extensive purification. Composite antibody-AAV nanoparticles structurally separate tropism and payload encapsulation, allowing each to be engineered independently.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

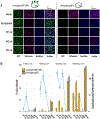
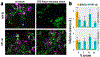



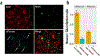
Similar articles
-
An Alternate Route for Adeno-associated Virus (AAV) Entry Independent of AAV Receptor.J Virol. 2018 Mar 14;92(7):e02213-17. doi: 10.1128/JVI.02213-17. Print 2018 Apr 1. J Virol. 2018. PMID: 29343568 Free PMC article.
-
Controlling AAV Tropism in the Nervous System with Natural and Engineered Capsids.Methods Mol Biol. 2016;1382:133-49. doi: 10.1007/978-1-4939-3271-9_10. Methods Mol Biol. 2016. PMID: 26611584 Free PMC article.
-
Mutational analysis of the adeno-associated virus type 2 (AAV2) capsid gene and construction of AAV2 vectors with altered tropism.J Virol. 2000 Sep;74(18):8635-47. doi: 10.1128/jvi.74.18.8635-8647.2000. J Virol. 2000. PMID: 10954565 Free PMC article.
-
Designer gene delivery vectors: molecular engineering and evolution of adeno-associated viral vectors for enhanced gene transfer.Pharm Res. 2008 Mar;25(3):489-99. doi: 10.1007/s11095-007-9431-0. Epub 2007 Sep 1. Pharm Res. 2008. PMID: 17763830 Free PMC article. Review.
-
Receptor targeting of adeno-associated virus vectors.Gene Ther. 2003 Jul;10(14):1142-51. doi: 10.1038/sj.gt.3301976. Gene Ther. 2003. PMID: 12833123 Review.
Cited by
-
Adeno-Associated Virus Engineering and Load Strategy for Tropism Modification, Immune Evasion and Enhanced Transgene Expression.Int J Nanomedicine. 2024 Jul 29;19:7691-7708. doi: 10.2147/IJN.S459905. eCollection 2024. Int J Nanomedicine. 2024. PMID: 39099791 Free PMC article. Review.
-
Fantastic AAV Gene Therapy Vectors and How to Find Them-Random Diversification, Rational Design and Machine Learning.Pathogens. 2022 Jul 3;11(7):756. doi: 10.3390/pathogens11070756. Pathogens. 2022. PMID: 35890005 Free PMC article. Review.
-
Protein Carrier Adeno-Associated Virus.ACS Nano. 2025 Apr 1;19(12):12308-12322. doi: 10.1021/acsnano.5c01498. Epub 2025 Mar 21. ACS Nano. 2025. PMID: 40117458 Free PMC article.
-
Coarse-Grained Simulations of Adeno-Associated Virus and Its Receptor Reveal Influences on Membrane Lipid Organization and Curvature.J Phys Chem B. 2024 Oct 17;128(41):10139-10153. doi: 10.1021/acs.jpcb.4c03087. Epub 2024 Oct 2. J Phys Chem B. 2024. PMID: 39356546 Free PMC article.
-
Synthetic Biology: Emerging Concepts to Design and Advance Adeno-Associated Viral Vectors for Gene Therapy.Adv Sci (Weinh). 2021 Feb 26;8(9):2004018. doi: 10.1002/advs.202004018. eCollection 2021 May. Adv Sci (Weinh). 2021. PMID: 33977059 Free PMC article. Review.
References
-
- Balakrishnan B, and Jayandharan GR (2014) Basic biology of adeno-associated virus (AAV) vectors used in gene therapy. Curr. Gene Ther 14, 86–100. - PubMed
-
- Smith RH (2008) Adeno-associated virus integration: virus versus vector. Gene Ther. 15, 817–822. - PubMed
-
- Dismuke DJ, Tenenbaum L, and Samulski RJ (2014) Biosafety of recombinant adeno-associated virus vectors. Curr. Gene Ther 13, 434–452. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

