Aryl Trehalose Derivatives as Vaccine Adjuvants for Mycobacterium tuberculosis
- PMID: 31809053
- PMCID: PMC6952572
- DOI: 10.1021/acs.jmedchem.9b01598
Aryl Trehalose Derivatives as Vaccine Adjuvants for Mycobacterium tuberculosis
Abstract
Mycobacterium tuberculosis (Mtb) continues to be a major health threat worldwide, and the development of Mtb vaccines could play a pivotal role in the prevention and control of this devastating epidemic. Th17-mediated immunity has been implicated in disease protection correlates of immune protection against Mtb. Currently, there are no approved adjuvants capable of driving a Th17 response in a vaccine setting. Recent clinical trial results using trehalose dibehenate have demonstrated a formulation-dependant proof of concept adjuvant system CAF01 capable of inducing long-lived protection. We have discovered a new class of Th17-inducing vaccine adjuvants based on the natural product Brartemicin. We synthesized and evaluated the capacity of a library of aryl trehalose derivatives to drive immunostimulatory reresponses and evaluated the structure-activity relationships in terms of the ability to engage the Mincle receptor and induce production of innate cytokines from human and murine cells. We elaborated on the structure-activity relationship of the new scaffold and demonstrated the ability of the lead entity to induce a pro-Th17 cytokine profile from primary human peripheral blood mononuclear cells and demonstrated efficacy in generating antibodies in combination with tuberculosis antigen M72 in a mouse model.
Figures
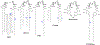








References
-
- WHO, Global tuberculosis report 2017. Geneva: World Health Organization; 2017; Licence: CC BY-NCSA, 3.0 IGO. WHO, Ed. 2017.
-
- Colditz GA; Brewer TF; Berkey CS; Wilson ME; Burdick E; Fineberg HV; Mosteller F, Efficacy of BCG Vaccine in the Prevention of Tuberculosis. Meta-analysis of the Published Literature. JAMA 1994, 271 (9), 698–702. - PubMed
-
- Andersen P; Doherty TM, The Success and Failure of BCG - Implications for a Novel Tuberculosis Vaccine. Nat. Rev. Microbiol 2005, 3 (8), 656–662. - PubMed
-
- Nemes E; Geldenhuys H; Rozot V; Rutkowski KT; Ratangee F; Bilek N; Mabwe S; Makhethe L; Erasmus M; Toefy A; Mulenga H; Hanekom WA; Self SG; Bekker L-G; Ryall R; Gurunathan S; DiazGranados CA; Andersen P; Kromann I; Evans T; Ellis RD; Landry B; Hokey DA; Hopkins R; Ginsberg AM; Scriba TJ; Hatherill M, Prevention of M. tuberculosis Infection with H4:IC31 Vaccine or BCG Revaccination. New England Journal of Medicine 2018, 379 (2), 138–149. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

